Tetrapoda: Acanthostega
Taxa on This Page
- Acanthostega
A Lot of Rot: the World of the Tetrapods
2. Hazardous Waste & Inefficiency: A Short History of Rubisco
[GO TO PART 1. An Introduction to Tetrapod Environments of the Late Devonian]
 It really isn't necessary to our plot line to explain the ultimate origins of the Late Devonian wood crisis, but the explanation makes rather compelling sense, to us at least. If we are correct, the Late Devonian wood problem was an almost inevitable result of evolutionary developments at the dawn of life.
It really isn't necessary to our plot line to explain the ultimate origins of the Late Devonian wood crisis, but the explanation makes rather compelling sense, to us at least. If we are correct, the Late Devonian wood problem was an almost inevitable result of evolutionary developments at the dawn of life.
As mentioned, the story begins deep in the Archean, even before the time of LUCA, the last universal common ancestor of all present life. At that time, all organisms were single cells, living in the sea, feeding on primordial soup, or by chemautotrophy, or by means of various other bizarre biochemical schemes which are found today, if at all, only in weird and extreme environments. However, all of these wildly varied cells, we are told, shared one common problem. They all faced death from a particularly noxious poison -- oxygen. Worse, in the high energy world of 2-3 Gy ago, oxygen would sometimes occur in highly reactive forms such as ozone (O3), hydrogen peroxide (H2O2), or even as some hideous epoxide or superoxide (O2-) radical. These nasty substances were virtually guaranteed to crosslink and denature proteins and cause all manner of metabolic and genetic havoc. Fortunately oxygen was, at the time, a very small component of the atmosphere. Still, it was found nearly everywhere in low concentration and had to be dealt with routinely. Consequently, one early development in the history of life was the evolution of a variety of enzymes whose basic function was to capture and destroy oxygen, and particularly reactive oxygen species, such as peroxides. These enzymes, not too much altered by evolution, are still common today. Examples include catalase and other peroxidases, such as those used to detoxify aberrant organic compounds in the tetrapod liver.
The material in the last paragraph is all standard textbook stuff with which we have taken no significant liberties. What we speculate is that, not long after LUCA, one group of bacteria developed an interesting variant on this system. Perhaps the whole thing started with one of those simple ion-exchange pumps so common in bacteria. The protein might let H+ into the cell down a concentration gradient, while pumping Mg++ out. Under appropriate conditions, magnesium ions can coordinate with oxygen, which would then be cotransported through the membrane and out of the cell. But, however it began, our supposition is that these cells evolved an enzyme which could use magnesium ions to coordinate free oxygen and a phosphorylated 5-carbon sugar (ribulose-1,5-biphosphate or RBP). Rather than transport the oxygen out of the cell, this enzyme could then react the oxygen with the RBP to yield (a) 3-phosphoglycerate (3PG) and (b) phosphoglycolate.
 You don't need to know what these molecules are at this point. It's enough to recognize that 3PG falls neatly into the routine metabolic pathways of the cell, a useful intermediate which can either be broken down for energy through glycolysis, or used as a brick to build glucose (e.g. for cell walls) or other complex sugars. Phosphoglycolate, on the other hand, is recycled to the amino acid glycine through a process which generates hazardous hydrogen peroxide. However, the glycolate is safe enough by itself. It can be transported to specialized peroxisomes by a sort of cellular bomb squad, for detonation under controlled conditions. Interestingly for us, the "explosive" reactivity of this peroxide is harnessed in the peroxisomes to degrade other toxic compounds, including aromatics (ring compounds with double bonds). Peroxide can detoxify these materials by derivatizing and cross-linking them for (a) disposal outside the cell, b) transformation into "friendly" aromatics like DNA bases and certain amino acids, or (c) degradation into digestible small molecules.
You don't need to know what these molecules are at this point. It's enough to recognize that 3PG falls neatly into the routine metabolic pathways of the cell, a useful intermediate which can either be broken down for energy through glycolysis, or used as a brick to build glucose (e.g. for cell walls) or other complex sugars. Phosphoglycolate, on the other hand, is recycled to the amino acid glycine through a process which generates hazardous hydrogen peroxide. However, the glycolate is safe enough by itself. It can be transported to specialized peroxisomes by a sort of cellular bomb squad, for detonation under controlled conditions. Interestingly for us, the "explosive" reactivity of this peroxide is harnessed in the peroxisomes to degrade other toxic compounds, including aromatics (ring compounds with double bonds). Peroxide can detoxify these materials by derivatizing and cross-linking them for (a) disposal outside the cell, b) transformation into "friendly" aromatics like DNA bases and certain amino acids, or (c) degradation into digestible small molecules.
Now, supposing all this to be true, we can see that this useful enzyme needn't be overly efficient, since oxygen was only present in small quantities. However, it did need to be relatively ubiquitous, since oxygen could turn up anywhere. A few of you, who have studied the right biochemistry, will see where we're going. Such an enzyme, evolved to react with oxygen, might well also react with carbon dioxide, in which case the reaction would become: RBP + CO2 => 2(3PG). And, in such a case, we would recognize the enzyme to be ribulose- 1,5- biphosphate carboxylase, or Rubisco -- the most common enzyme on earth today. See also Eukarya glossary entry. Rubisco is the key enzyme in photosynthesis. It is this enzyme which actually takes atmospheric carbon dioxide and incorporates it into sugars. Thus, our speculation is not really so arbitrary after all. We have simply postulated that rubisco began life with a different function. (Nor are we the first to make guesses of this sort! See, e.g., Introduction)
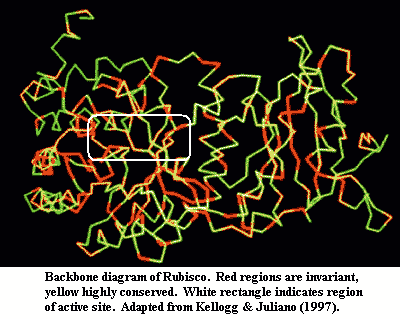
 Why do we think so? Rubisco has two characteristics which have puzzled biochemists for years. First, it is one of life's least efficient enzymes. It works so slowly that enormous quantities are needed to get the job done in today's atmosphere. Second, it is incredibly sloppy and reacts with oxygen as well as CO2, forming messy glycoylate which has to be recycled at some expense and danger to the cell, as described above. Frankly, this only makes sense if the original function were to dispose of oxygen, carbon dioxide being tolerated as an alternate substrate because it did no harm. It bears emphasis that atmospheric oxygen was very low, and carbon dioxide very high, for a very long time. From an evolutionary point of view, oxidative metabolism is relatively recent. Even after the evolution of photosynthesis, rubisco may have had both functions, since the newly evolved cyanobacteria needed to have a method for disposing of the waste oxygen generated by photosynthesis, before it reached dangerous intracellular levels. Thus, it is unsurprising that rubisco still reacts with both oxygen and carbon dioxide. It evolved under selective pressure to perfect this dual ability for two or three billion years, without much selection for efficiency. There are strong experimental indications that rubisco is, by now, under very tight genetic constraint, and has little freedom to evolve into some hypothetical faster or more selective form more consistent with Cenozoic requirements. See, e.g., Kellogg & Juliano (1997); Leebens-Mack & dePamphilis (2002).
Why do we think so? Rubisco has two characteristics which have puzzled biochemists for years. First, it is one of life's least efficient enzymes. It works so slowly that enormous quantities are needed to get the job done in today's atmosphere. Second, it is incredibly sloppy and reacts with oxygen as well as CO2, forming messy glycoylate which has to be recycled at some expense and danger to the cell, as described above. Frankly, this only makes sense if the original function were to dispose of oxygen, carbon dioxide being tolerated as an alternate substrate because it did no harm. It bears emphasis that atmospheric oxygen was very low, and carbon dioxide very high, for a very long time. From an evolutionary point of view, oxidative metabolism is relatively recent. Even after the evolution of photosynthesis, rubisco may have had both functions, since the newly evolved cyanobacteria needed to have a method for disposing of the waste oxygen generated by photosynthesis, before it reached dangerous intracellular levels. Thus, it is unsurprising that rubisco still reacts with both oxygen and carbon dioxide. It evolved under selective pressure to perfect this dual ability for two or three billion years, without much selection for efficiency. There are strong experimental indications that rubisco is, by now, under very tight genetic constraint, and has little freedom to evolve into some hypothetical faster or more selective form more consistent with Cenozoic requirements. See, e.g., Kellogg & Juliano (1997); Leebens-Mack & dePamphilis (2002).
During the Archean and Early Proterozoic, continental iron deposits and reduced sulfur species acted as a gigantic sink, sopping up excess oxygen and keeping things almost balanced. Oxygen levels increased only slowly, incrementally. That gave life time to develop oxidative metabolic pathways. Eventually, oxygen was no longer a poison, and became an indispensible metabolite. But, fast forward to the Devonian. Plants were now moving onto land in a big way. This had been happening, very slowly, since the Late Ordovician. Refer to the atmosphere graph on the previous page. The early downward drift of carbon dioxide levels may be explained as the gradual accumulation of a standing crop of plant biomass in and around fresh waters and tidal regions. However, full terrestriality was achieved in the Devonian; and, with it, a sudden rise in atmospheric oxygen. What would (or wood) happen then? ATW050703.
Images: The Archean scene is from NASA. I'm uncertain where the Devonian scene is from. I found it here.
Acanthostega, were it not already extinct, would have been beaten to death over the last 15 years or so by three of paleontology's best minds and pens, and we will not attempt to improve on the excellent state of the literature. From time to time we comment on science in the manner of art critics; and from that point of view, it's a real treat to read and compare the styles of Clack, Ahlberg and Coates. We have already commented on Ahlberg's style, a cross between Baron Cuvier and David Copperfield. Coates is very different. He publishes relatively little, but his principal papers are each massive, thorough, and intricately detailed. Coates (1996) is still probably the best single paper on any early tetrapod ever published. It is now a little dated in some areas. However, there is yet unexploited material for any number of follow-up studies based on the insights he generated through exhaustive comparative analysis of the early tetrapod material.
If we had to pick, Clack might have the most athletic mind of the three. One gets the impression that she has to force herself to quit working long enough to write things down. Each paper is a work in progress -- "we're still working on this," "we look forward to getting the results of that project." She changes her mind more often than most scientists and delights in explaining where she went wrong last time and how much progress is being made. She is also the most likely of the three to go shooting off in some new direction to develop a new angle, e.g., the neuroanatomy of hearing, the mechanics of underwater locomotion, or the physiology of respiration.
They make a remarkable group, and seem to be attracting an equally talented new generation. Of these, we take particularly unmerited pleasure in mentioning Dr. Henning Blom, now in Per Ahlberg's lab. Our unearned satisfaction is derived solely from the fact that we spotted Blom as a name to watch, based on his thelodont work, in the late 1990's. If we could only spot new basketball talent in the same manner ... . In any event, and despite Prof. Clack's frustrating inability to muscle in a lay-up past the likes of Yao Ming, we are extremely lucky have these folks on hand to deal with Acanthostega. We nearly did not -- but we will deal with some of that history, very briefly, when we get to Ichthyostega. ATW050704.
Descriptions
 Acanthostega: A. gunnari Jarvik, 1952.
Acanthostega: A. gunnari Jarvik, 1952.
Range: Late Devonian (Famennian) of East Greenland (Aina Dal Fm. & Britta Dal Fm.) Possibly very early Famennian, based on co-occurrence of Phyllolepis and Remigolepis. Long & Gordon (2004).
Phylogeny: Tetrapoda : (Ichthyostega + Sinostega + (Densignathus + (Hynerpeton + (Tulerpeton + (Ossinodus + (Whatcheeriidae + (Crassigyrinus + (Colosteidae + (Spathicephalus + (Baphetidae + Tetrapoda*))))))))))) + *.
Characters: image of life reconstruction;
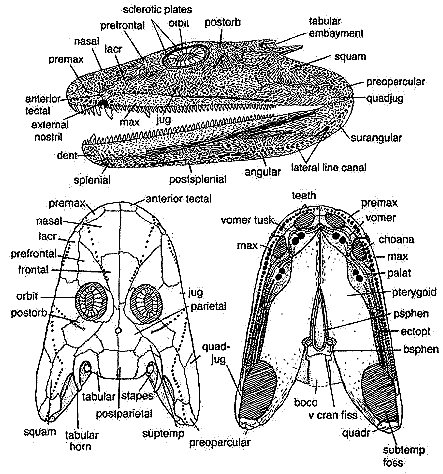
dermal skull: additional image of skull [Cl02] and discussion; paired median rostrals [Cl98a]; superficial anterior tectal above nares [Cl98a]; external nostrils small & close to jaw margin; naris and choana may have been used for chemosensation (as osteolepiforms?); premaxilla not sutured to maxilla [LCl93] and loosely sutured to nasals [Cl98a]; spade-shaped snout with enlarged bilateral nasals [Co96]; nasal bones do not suture together in the midline, leaving internasal fontanelle as in Ventastega [Cl03]; additional gap between nasals and median rostrals [Cl03]; prefrontal-jugal contact excludes lacrimal from orbit [Cl02a] [R+03]; prefrontal elongate & triangular [Cl02a]; postfrontal large, very thick [Cl02a]; "arrow-shaped supratemporal spanning skull table-cheek junction" [$Co96] [$Cl02a]; intertemporal absent [LCl93] [R+03]; postparietals forming square, but weakly sutured together [Cl02a]; tabular with both a posteriorly directed horn & embayment with possible spiracle [$Co96] [$Cl98a] [R+03]; eyes supported by ring of sclerotic plates; orbits enlarged relative to osteolepiforms [A98]; deep postorbital participating in orbit [A98] [LG04]; jugal large and extends anterior to orbit, underlapping all surrounding bones except maxilla [Cl02a] [R+03]; squamosal large, with hook-like process clasping tabular [Cl02a]; possible soft operculum attached to squamosal [LG04]; quadratojugal elongate triangle with narrow tapering process separating jugal from jaw margin [Cl02a];
branchial: preoperculars present [Co96]; large ceratohyal & 3+ well-developed & deeply grooved branchial region (i.e. functional gills) [CoCl91] [Co96] [Cl+03]; operculogular series absent [Co96];
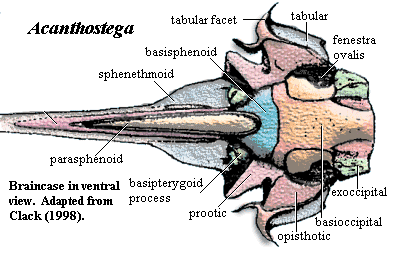 Occiput & braincase: exoccipitals small; braincase enclosing notochord; otic region short, dorsally flat & square [Cl+03]; otic region, ventral part unossified [Cl98a]; opisthotic and prootic fused [Cl]98; opisthotic forms crista parotica laterally, probable unossified posterior facet for exoccipital, and ventrally forms anterior wall of lateral otic fissure [Cl98a]; lateral otic fissure continuous with fenestra vestibulae [Co96]; opisthotic posterolateral corner with slight groove for lateral head vein and probably part of jugular foramen [Cl98a]; crista parotica shelf forms posterodorsal margin of fenestra vestibuli [Cl98a]; stapes strongly attached to fenestra ovalis [Cl98a]; fenestra vestibulae large, accommodating stapedial head; stapes short & stout [LG04]; stapes with large proximal footplate & flared distal end; image of lateral braincase [Cl98a] and discussion; stapes incorporating both heads of former hyomandibula, visible as two closely appressed semicircles [Cl94]; margins of fenestra vestibuli formed by basioccipital & otic capsule, excluding basisphenoid and parasphenoid [Cl94] [Co96]; lateral commisure absent [Co96]; prootic robust, with sidewalls enclosing semicircular canals [Cl98a]; anterior prootic forming possible spiracular groove continuous with spiracular notch in tabular [Cl98a]; basisphenoid without notochordal pit [Cl98a]; basisphenoid with dorsally directed dorsum sellae "wings" weakly sutured to prootic & basioccipital [Cl98a]; ventral cranial fissure sutured, but traceable [R+03]; parasphenoid not contacting basisphenoid or prootic [Cl98a]; epipterygoid not contacting otic capsule [Cl94]; basipterygoid processes "bifaceted, cartilage-finished, apparently [with] synovial surfaces [and] approximately semicircular and slightly concave. The processes project a little below the parasphenoid. They are large, conspicuous, bulbous structures in ventral view, unlike those of any non-tetrapod osteichthyan, [and] were large and bifaceted." [Cl98a]; sphenethmoid region weakly attached to otoccipital [Cl98a]; sphenethmoid very lightly ossified, with nasal capsules unossified or not recovered [Cl98a]; sphenethmoid fused with basisphenoid [Cl98a]; sphenethmoid with muscle scar for orbital retractors [Cl98a]; sphenethmoid narrows abruptly anteriorly (primitive), with pineal foramen on broader posterior part (derived) [Cl98a];
Occiput & braincase: exoccipitals small; braincase enclosing notochord; otic region short, dorsally flat & square [Cl+03]; otic region, ventral part unossified [Cl98a]; opisthotic and prootic fused [Cl]98; opisthotic forms crista parotica laterally, probable unossified posterior facet for exoccipital, and ventrally forms anterior wall of lateral otic fissure [Cl98a]; lateral otic fissure continuous with fenestra vestibulae [Co96]; opisthotic posterolateral corner with slight groove for lateral head vein and probably part of jugular foramen [Cl98a]; crista parotica shelf forms posterodorsal margin of fenestra vestibuli [Cl98a]; stapes strongly attached to fenestra ovalis [Cl98a]; fenestra vestibulae large, accommodating stapedial head; stapes short & stout [LG04]; stapes with large proximal footplate & flared distal end; image of lateral braincase [Cl98a] and discussion; stapes incorporating both heads of former hyomandibula, visible as two closely appressed semicircles [Cl94]; margins of fenestra vestibuli formed by basioccipital & otic capsule, excluding basisphenoid and parasphenoid [Cl94] [Co96]; lateral commisure absent [Co96]; prootic robust, with sidewalls enclosing semicircular canals [Cl98a]; anterior prootic forming possible spiracular groove continuous with spiracular notch in tabular [Cl98a]; basisphenoid without notochordal pit [Cl98a]; basisphenoid with dorsally directed dorsum sellae "wings" weakly sutured to prootic & basioccipital [Cl98a]; ventral cranial fissure sutured, but traceable [R+03]; parasphenoid not contacting basisphenoid or prootic [Cl98a]; epipterygoid not contacting otic capsule [Cl94]; basipterygoid processes "bifaceted, cartilage-finished, apparently [with] synovial surfaces [and] approximately semicircular and slightly concave. The processes project a little below the parasphenoid. They are large, conspicuous, bulbous structures in ventral view, unlike those of any non-tetrapod osteichthyan, [and] were large and bifaceted." [Cl98a]; sphenethmoid region weakly attached to otoccipital [Cl98a]; sphenethmoid very lightly ossified, with nasal capsules unossified or not recovered [Cl98a]; sphenethmoid fused with basisphenoid [Cl98a]; sphenethmoid with muscle scar for orbital retractors [Cl98a]; sphenethmoid narrows abruptly anteriorly (primitive), with pineal foramen on broader posterior part (derived) [Cl98a];
palate: anterior palatal vacuity paired [R+03]; pterygoids covered with shagreen [Cl02a]; ectopterygoid forms significant part of adductor fossa [Cl02a]; parasphenoid terminates anterior to ventral cranial fissure [Co96];
jaw: lower jaw slender, long & low, with shallow ventral curvature [ACl98]; dentary narrow and tapers to a point posteriorly, not reaching articular [ACl98]; dentary weakly sutured to rest of jaw [ACl98]; dentary crest present, but without accessory tooth row [ACl98]; infradentaries with broad lateral exposure and sharp ventral margin [ACl98]; dorsal angular crest absent [ACl98]; angular contacts prearticular [R+03$] surangular rhomboidal & plate-like [ACl98]; surangular almost covers articular laterally and posteriorly [ACl98]; 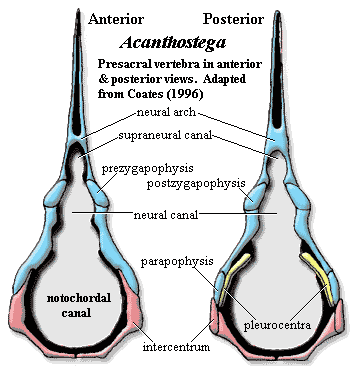 surangular participates in adductor fossa [ACl98]; Meckelian unossified between prearticular and dentaries [ACl98]; "foraminal" parasymphysial dental plate present [Co96]; medial parasymphysial foramen present, but lateral foramen absent (compare Obruchevichthys) [ACl98]; splenial, mesial lamina well-developed as strong buttress below parasymphysial plate [ACl98]; splenial with posterior process overlapping prearticular [ACl98]; muscle attachment striations on buttress & posterior process [ACl98]; splenial ventral edge forming deep concave space on medial face [ACl98]; "The hollow tube formed by the splenial, dentary and parasymphysial plate is occupied in part by remnants of Meckelian bone" [ACl98]; splenial not sutured to anterior coronoid [R+03]; prearticular very large, extending from articular almost to symphysis, with dorsal ridge bearing band of shagreen [ACl98]; prearticular not sutured ventrally to surangular, angular or postsplenial; prearticular lower margin with small Meckelian fossae; prearticular forms medial margin of adductor fossa [ACl98]; prearticular with tight suture to all coronoids and parasymphysial plate [ACl98];
surangular participates in adductor fossa [ACl98]; Meckelian unossified between prearticular and dentaries [ACl98]; "foraminal" parasymphysial dental plate present [Co96]; medial parasymphysial foramen present, but lateral foramen absent (compare Obruchevichthys) [ACl98]; splenial, mesial lamina well-developed as strong buttress below parasymphysial plate [ACl98]; splenial with posterior process overlapping prearticular [ACl98]; muscle attachment striations on buttress & posterior process [ACl98]; splenial ventral edge forming deep concave space on medial face [ACl98]; "The hollow tube formed by the splenial, dentary and parasymphysial plate is occupied in part by remnants of Meckelian bone" [ACl98]; splenial not sutured to anterior coronoid [R+03]; prearticular very large, extending from articular almost to symphysis, with dorsal ridge bearing band of shagreen [ACl98]; prearticular not sutured ventrally to surangular, angular or postsplenial; prearticular lower margin with small Meckelian fossae; prearticular forms medial margin of adductor fossa [ACl98]; prearticular with tight suture to all coronoids and parasymphysial plate [ACl98];
dentition: vomer with large fang-pairs; palatines & ectopterygoids with marginal row of smaller teeth & denticles; all coronoids with reduced dentition consisting of row of small teeth and denticles similar to palatal bones, but fang-pairs absent lCl02a]; anterior coronoid fang pair offset from tooth row [LCl93]; maxilla with ~40 teeth, premaxilla with ~13 [Co96]; dentary with smaller and more numerous teeth (about 75 [Co96: ~70] than upper jaw (about 60) and anterior fang pair [ACl98]; well-ossified gill arches;
axial skeleton: 28 to 30 notochordal, rhachitomous presacral vertebrae, with neural arches sometimes remaining as paired structures [A98] [compare Co96: 30+ presacrals]; little regional specialization in presacral vertebrae [Co96]; intercentra paired, with complete ventral fusion only in atlas & sacral intercentra [Co96]; pleurocentra not well ossified (unlike Ichthyostega) [Co96]; neural arches with canals for both nerve cord and a (more ventral!?) supraneural ligament [A98]; small atlas arches set over large atlantal intercentrum; post-atlas arches little differentiated; narrow zygapophyses present but poorly developed [Co96] [Ca+05]; transverse processes & diapophyses only very slightly developed [Co96]; arches weakly bound to centra [Co96]; neural spines squared off [Co96]; accessory articulations present between some neural spines; ribs relatively short, straight, & slight, present from atlas to caudal #4 [Co96]; anterior thoracic & posterior cervical ribs uncinate [Co96]; rib articulations directed posterolaterally, dorsally continuous with posterior intercentral rim [Co96]; ribs with broadly spatulate proximal end, not conspicuously bifurcated [Co96]; anterior thoracic ribs distally expanded [Co96]; pelvis attached to vertebral  column via single pair of elongate sacral ribs [Co96] [Ca+05]; sacral centrum not strongly differentiated [Co96]; deep tail supported by fin rays and accessory internal supports; about 35 caudal vertebrae [Co96]; caudal fin more extensive than in Ichthyostega [Co96]; caudal intercentra fused from caudal #4 [Co96]; caudals 1-4 with ribs [Co96]; hemal arches, fused to intercentra, begin at caudal #4 [Co96]; "first three complete hemal arches (caudal intercentra 5-7) are specialized and fit closely together" [Co96] [3]; caudal #6 with anteriorly serrated hemal arch plus spine [$Co96]; supraneural canal absent in the caudal region [Co96]; caudal fin lepidotrichia present, elongate, unsegmented, and unbranched [Co96]; first neural radial from 8th caudal vertebra, and first hemal radial 15th caudal vertebra [$Co96]; caudal supraneural spines articulated with some arches dorsally, to support the tailfin;
column via single pair of elongate sacral ribs [Co96] [Ca+05]; sacral centrum not strongly differentiated [Co96]; deep tail supported by fin rays and accessory internal supports; about 35 caudal vertebrae [Co96]; caudal fin more extensive than in Ichthyostega [Co96]; caudal intercentra fused from caudal #4 [Co96]; caudals 1-4 with ribs [Co96]; hemal arches, fused to intercentra, begin at caudal #4 [Co96]; "first three complete hemal arches (caudal intercentra 5-7) are specialized and fit closely together" [Co96] [3]; caudal #6 with anteriorly serrated hemal arch plus spine [$Co96]; supraneural canal absent in the caudal region [Co96]; caudal fin lepidotrichia present, elongate, unsegmented, and unbranched [Co96]; first neural radial from 8th caudal vertebra, and first hemal radial 15th caudal vertebra [$Co96]; caudal supraneural spines articulated with some arches dorsally, to support the tailfin;
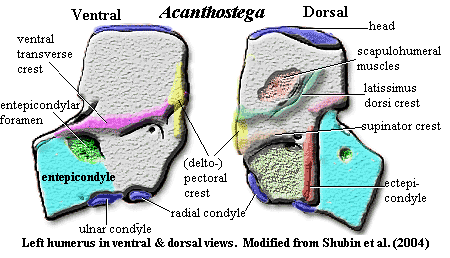 appendicular skeleton: shoulder girdle detached from skull [Co96], with loss of posttemporals and supracleithra [LG04]; pectoral girdle image [Co96] [Co+02] & further discussion; cleithrum & scapulocoracoid co-ossified as Ichthyostega and Hynerpeton); dorsal anocleithrum retained (as Tulerpeton) [Co96] [R+03]; an incurved flange (= postbranchial lamina) of cleithrum runs down leading edge of shoulder girdle (in fishes this lamina forms the back of the opercular chamber and helps direct water out of it) [CoCl91] [Co96] [L+00]; anteroventral process of cleithrum wraps around scapulocoracoid [A98]; numerous scapulocoracoid foramina [LCo95]; endochondral bone in coracoid region very thin [Co96]; See Atlas & Gazetteer for further details on the scapulocoracoid and cleithrum; clavicles with "broad, rounded subtriangular ventral plate and a rod-like ascending, dorsal process" [Co96]; clavicles do not meet anteriorly [R+03]; posterior margin of interclavicle drawn out into parasternal process [R+03]; large glenoid foramen [R+03]; glenoid posterolaterally oriented, with strap-shaped, strongly concave fossa slightly helical ('screw-shaped') [Co96]; humerus L-shaped and very flat, with accessory foramina as in Ichthyostega [S+04]; latissimus dorsi process offset anteriorly [LCo95] [2] [R+03]; humerus, anterior edge with near-vertical deltopectoral crest [LCo95] [4]; "recess and incipient crest at the proximal union of ectepicondylar and entepicondylar processes" [S+04]; humerus with transverse ventral ridge [S+04]; entepicondyle somewhat rectangular [Co96]; distally extended ectepicondylar process [S+04]; extensive extensor muscle scars on posterior side of ectepicondyle extending onto entepicondyle [Co96]; epipodial facets face laterally (distally) [Co96]; radial & ulnar facets face distally (not ventrally as in later tetrapods) [S+04]; convex facets well-separated [LCo95]; radial facet bimodal (lateral and anteriorly directed surfaces) [Co96]; epipodials shaped as triangular prism, with distinct, straight anterior surface, and sharp, convex posterior edge [Co96]; radius much longer than triangular ulna (as in Eusthenopteron); radius distally spatulate and flat [Co96]; olecranon process absent [Co96]; 'elbow' joint held more or less straight; 'wrist' diffuse structure formed around arc encompassing the long radius and short ulna [Ca+05]; distinct carpal (intermedium) & tarsals [Co96] [Ca+05]; intermedium articulates only proximally & distally [Co+02]; eight manual digits [Ca+05]; manus with phalangeal formula of 33334443 [$Co96]; paddle-like limbs; individual phalanges cylindrical and slightly constricted in the middle [Co96]; dermal fin rays absent [Co+02];
appendicular skeleton: shoulder girdle detached from skull [Co96], with loss of posttemporals and supracleithra [LG04]; pectoral girdle image [Co96] [Co+02] & further discussion; cleithrum & scapulocoracoid co-ossified as Ichthyostega and Hynerpeton); dorsal anocleithrum retained (as Tulerpeton) [Co96] [R+03]; an incurved flange (= postbranchial lamina) of cleithrum runs down leading edge of shoulder girdle (in fishes this lamina forms the back of the opercular chamber and helps direct water out of it) [CoCl91] [Co96] [L+00]; anteroventral process of cleithrum wraps around scapulocoracoid [A98]; numerous scapulocoracoid foramina [LCo95]; endochondral bone in coracoid region very thin [Co96]; See Atlas & Gazetteer for further details on the scapulocoracoid and cleithrum; clavicles with "broad, rounded subtriangular ventral plate and a rod-like ascending, dorsal process" [Co96]; clavicles do not meet anteriorly [R+03]; posterior margin of interclavicle drawn out into parasternal process [R+03]; large glenoid foramen [R+03]; glenoid posterolaterally oriented, with strap-shaped, strongly concave fossa slightly helical ('screw-shaped') [Co96]; humerus L-shaped and very flat, with accessory foramina as in Ichthyostega [S+04]; latissimus dorsi process offset anteriorly [LCo95] [2] [R+03]; humerus, anterior edge with near-vertical deltopectoral crest [LCo95] [4]; "recess and incipient crest at the proximal union of ectepicondylar and entepicondylar processes" [S+04]; humerus with transverse ventral ridge [S+04]; entepicondyle somewhat rectangular [Co96]; distally extended ectepicondylar process [S+04]; extensive extensor muscle scars on posterior side of ectepicondyle extending onto entepicondyle [Co96]; epipodial facets face laterally (distally) [Co96]; radial & ulnar facets face distally (not ventrally as in later tetrapods) [S+04]; convex facets well-separated [LCo95]; radial facet bimodal (lateral and anteriorly directed surfaces) [Co96]; epipodials shaped as triangular prism, with distinct, straight anterior surface, and sharp, convex posterior edge [Co96]; radius much longer than triangular ulna (as in Eusthenopteron); radius distally spatulate and flat [Co96]; olecranon process absent [Co96]; 'elbow' joint held more or less straight; 'wrist' diffuse structure formed around arc encompassing the long radius and short ulna [Ca+05]; distinct carpal (intermedium) & tarsals [Co96] [Ca+05]; intermedium articulates only proximally & distally [Co+02]; eight manual digits [Ca+05]; manus with phalangeal formula of 33334443 [$Co96]; paddle-like limbs; individual phalanges cylindrical and slightly constricted in the middle [Co96]; dermal fin rays absent [Co+02];
pelvic: image of pelvis [Co96] and discussion; pelvis attached to vertebral column via sacral rib [Ca+05]; no distinct facet for sacral rib [Co96]; pelvis is single ossification with no sutures [Co96]; pelvic blades all smooth except extensive ventromedial "striations extending onto the base of the dorsal iliac process" (probably = iliofemoralis insertion) [Co96]; biramous ilium [Co96]; pelvic post-iliac process long & posterodorsally directed with upright-oval cross-section [A98]; iliac neck attached to pelvis posterior to acetabulum [Co96] [A98]; postacetabular buttress more prominent than supra-acetabular buttress [Co96]; pubo-ischial pelvic symphysis [Co96] [Co+02]; pelvic plate posterior to acetabulum thinly ossified [Co96]; image of hindlimb [Co96]; hindlimb was paddle-like similar to Ichthyostega; femur 25% longer than humerus [Co96] (compare [R+03]: femur approximately same length as humerus); femur relatively slender with large rectangular adductor blade placed midway along its length [LCo95] [Co96]; "internal trochanter is separated from the femoral head and projects proximally above a short smooth groove" [LCo95]; femur with ~75° anterior tortion [LCo95] [Co96] [A98$?]; "proximodorsal extensor) surface is convex and smooth" [Co96]; tibial facet on anterior condyle only & fibular facet on posterior condyle [Co96]; tibia 25% longer than fibula [Co96]; tibia blocky & rectangular [A98]; tibia and fibula flattened and overlapped each other slightly in life in a manner suitable for twisting in a swimming stroke but not for bending at the 'knee'; tibia with "well-developed cnemial crest, flanked anteriorly by a series of muscle scars" [LCo95]; ridge present near posterior edge of flexor surface of fibula [R+03]; fibulare articulates directly with digits [Co96]; 'ankle' consisted of a few flattened tarsals, with no obvious ankle joint; 8+ pedal digits with formula probably 1,2,3,3,3.3,3,2 [Co96] [L+00];
integument: ventral scales and gastralia, but no evidence of dorsal scales [Co96]; thinly ossified scutes associated with undetermined limb elements [Co96]; lateral line contained in tubes running through dermal bones, opening by series of pores;
other: perhaps an entirely aquatic organism [Cl02] or perhaps not [Ca+05].
Images: Skull reconstructions from Clack 2002). Photograph from Prof. Clack's website. Images elsewhere on this site include life reconstruction,additional image of skull [Cl02], occipital region [B00]; image of lateral braincase [Cl98a], pectoral girdle image [Co96] [Co+02], image of pelvis [Co96], image of hindlimb [Co96].
 Notes: [1] Clack, on the Tree of Life page, notes that stapes formed the only bony link between the braincase and the palate, apart from the basal articulation, and may have acted as a brace between the two. It may also have provided an origin for spiracle-operating muscles. [2] "(misidentified as a deltoid process in Coates & Clack, 1990)" [LCo95: 315]. [3] The details are complex, but the point is that there is a "break" in the tail here, probably permitting the tail to move without jerking the entire spine around. Whales, for example have a group of specialized, highly mobile vertebrae just anterior to the fluke. [4] Coates [Co96] notes that his identification of the latissimus dorsi process is at odds with the Andrews & Westoll (1970) interpretation of Eusthenopteron. [AW70] identify the analogous structure in Eusthenopteron as a deltoid process. For what it may be worth, we strongly favor the Coates interpretation. Coates also sounds an apt note of warning that, although the various humeral structures are likely homologous to the attachment sites for the muscles for which they are named, "[i]nterpretations of musculature for extremely primitive limb skeletons need to be treated with caution, because the degree of muscular differentiation which had evolved from that of paired sarcopterygian fins is most uncertain." [Co96: 383-85].
Notes: [1] Clack, on the Tree of Life page, notes that stapes formed the only bony link between the braincase and the palate, apart from the basal articulation, and may have acted as a brace between the two. It may also have provided an origin for spiracle-operating muscles. [2] "(misidentified as a deltoid process in Coates & Clack, 1990)" [LCo95: 315]. [3] The details are complex, but the point is that there is a "break" in the tail here, probably permitting the tail to move without jerking the entire spine around. Whales, for example have a group of specialized, highly mobile vertebrae just anterior to the fluke. [4] Coates [Co96] notes that his identification of the latissimus dorsi process is at odds with the Andrews & Westoll (1970) interpretation of Eusthenopteron. [AW70] identify the analogous structure in Eusthenopteron as a deltoid process. For what it may be worth, we strongly favor the Coates interpretation. Coates also sounds an apt note of warning that, although the various humeral structures are likely homologous to the attachment sites for the muscles for which they are named, "[i]nterpretations of musculature for extremely primitive limb skeletons need to be treated with caution, because the degree of muscular differentiation which had evolved from that of paired sarcopterygian fins is most uncertain." [Co96: 383-85].
Links: Acanthostega gunnari; Devonian Times - about Acanthostega; \Acanthostega\ by Janice McCafferty; Québec Science - L'acanthostega, notre nouvel ancêtre; Acanthostega; Acanthostega: fossil. . .; acanthostega.htm; Acanthostega gunnari; NOVA Online | The Missing Link | Diva of the Devonian (3) | PBS; 256.htm; Zimmer Chapter Four; Fall'96Syllabus; Biology 356; DEVONIANO Tetrápodes 1; Devoniano Portuguese); Biology 356.
References: Ahlberg (1998) [A98]; Ahlberg & Clack (1998) [ACl98]; Andrews & Westoll (1970) [AW70]; Berman (2000) [B00]; Carroll et al. (2005) [Ca+05]; Clack (1994) [Cl94]; Clack (1998a) [Cl98a]; Clack (2002) [Cl02]; Clack 2002a) [Cl02a]; Clack (2003) [Cl03]; Clack et al. (2003) [Cl+03]; Coates (1996) [Co96]; Coates & Clack (1991) [CoCl91]; Coates et al. 2002) [Co+02]; Laurin et al. (2000) [L+00]; Lebedev & Coates (1995) [LCo95]; Long & Gordon (2004) [LG04]; Ruta et al. (2003) [R+03]; Shubin et al. (2004) [S+04]. ATW050702.
 It really isn't necessary to our plot line to explain the ultimate origins of the Late Devonian wood crisis, but the explanation makes rather compelling sense, to us at least. If we are correct, the Late Devonian wood problem was an almost inevitable result of evolutionary developments at the dawn of life.
It really isn't necessary to our plot line to explain the ultimate origins of the Late Devonian wood crisis, but the explanation makes rather compelling sense, to us at least. If we are correct, the Late Devonian wood problem was an almost inevitable result of evolutionary developments at the dawn of life. You don't need to know what these molecules are at this point. It's enough to recognize that 3PG falls neatly into the routine metabolic pathways of the cell, a useful intermediate which can either be broken down for energy through glycolysis, or used as a brick to build glucose (e.g. for cell walls) or other complex sugars. Phosphoglycolate, on the other hand, is recycled to the amino acid glycine through a process which generates hazardous hydrogen peroxide. However, the glycolate is safe enough by itself. It can be transported to specialized peroxisomes by a sort of cellular bomb squad, for detonation under controlled conditions. Interestingly for us, the "explosive" reactivity of this peroxide is harnessed in the peroxisomes to degrade other toxic compounds, including aromatics (ring compounds with double bonds). Peroxide can detoxify these materials by derivatizing and cross-linking them for (a) disposal outside the cell, b) transformation into "friendly" aromatics like DNA bases and certain amino acids, or (c) degradation into digestible small molecules.
You don't need to know what these molecules are at this point. It's enough to recognize that 3PG falls neatly into the routine metabolic pathways of the cell, a useful intermediate which can either be broken down for energy through glycolysis, or used as a brick to build glucose (e.g. for cell walls) or other complex sugars. Phosphoglycolate, on the other hand, is recycled to the amino acid glycine through a process which generates hazardous hydrogen peroxide. However, the glycolate is safe enough by itself. It can be transported to specialized peroxisomes by a sort of cellular bomb squad, for detonation under controlled conditions. Interestingly for us, the "explosive" reactivity of this peroxide is harnessed in the peroxisomes to degrade other toxic compounds, including aromatics (ring compounds with double bonds). Peroxide can detoxify these materials by derivatizing and cross-linking them for (a) disposal outside the cell, b) transformation into "friendly" aromatics like DNA bases and certain amino acids, or (c) degradation into digestible small molecules. Why do we think so? Rubisco has two characteristics which have puzzled biochemists for years. First, it is one of life's least efficient enzymes. It works so slowly that enormous quantities are needed to get the job done in today's atmosphere. Second, it is incredibly sloppy and reacts with oxygen as well as CO2, forming messy glycoylate which has to be recycled at some expense and danger to the cell, as described above. Frankly, this only makes sense if the original function were to dispose of oxygen, carbon dioxide being tolerated as an alternate substrate because it did no harm. It bears emphasis that atmospheric oxygen was very low, and carbon dioxide very high, for a very long time. From an evolutionary point of view, oxidative metabolism is relatively recent. Even after the evolution of photosynthesis, rubisco may have had both functions, since the newly evolved cyanobacteria needed to have a method for disposing of the waste oxygen generated by photosynthesis, before it reached dangerous intracellular levels. Thus, it is unsurprising that rubisco still reacts with both oxygen and carbon dioxide. It evolved under selective pressure to perfect this dual ability for two or three billion years, without much selection for efficiency. There are strong experimental indications that rubisco is, by now, under very tight genetic constraint, and has little freedom to evolve into some hypothetical faster or more selective form more consistent with
Why do we think so? Rubisco has two characteristics which have puzzled biochemists for years. First, it is one of life's least efficient enzymes. It works so slowly that enormous quantities are needed to get the job done in today's atmosphere. Second, it is incredibly sloppy and reacts with oxygen as well as CO2, forming messy glycoylate which has to be recycled at some expense and danger to the cell, as described above. Frankly, this only makes sense if the original function were to dispose of oxygen, carbon dioxide being tolerated as an alternate substrate because it did no harm. It bears emphasis that atmospheric oxygen was very low, and carbon dioxide very high, for a very long time. From an evolutionary point of view, oxidative metabolism is relatively recent. Even after the evolution of photosynthesis, rubisco may have had both functions, since the newly evolved cyanobacteria needed to have a method for disposing of the waste oxygen generated by photosynthesis, before it reached dangerous intracellular levels. Thus, it is unsurprising that rubisco still reacts with both oxygen and carbon dioxide. It evolved under selective pressure to perfect this dual ability for two or three billion years, without much selection for efficiency. There are strong experimental indications that rubisco is, by now, under very tight genetic constraint, and has little freedom to evolve into some hypothetical faster or more selective form more consistent with  Acanthostega
Acanthostega
 Occiput & braincase: exoccipitals small; braincase enclosing notochord; otic region short, dorsally flat & square [Cl+03]; otic region, ventral part unossified [Cl98a]; opisthotic and prootic fused [Cl]98; opisthotic forms
Occiput & braincase: exoccipitals small; braincase enclosing notochord; otic region short, dorsally flat & square [Cl+03]; otic region, ventral part unossified [Cl98a]; opisthotic and prootic fused [Cl]98; opisthotic forms  surangular participates in adductor fossa [ACl98];
surangular participates in adductor fossa [ACl98];  column via single pair of elongate
column via single pair of elongate  appendicular skeleton: shoulder girdle detached from skull [Co96], with loss of
appendicular skeleton: shoulder girdle detached from skull [Co96], with loss of  Notes: [1] Clack, on the
Notes: [1] Clack, on the