|
|
Bones: Dermal Bones |
| The Vertebrates |
Facial Series:
Septomaxilla-2 |
The Facial Series: Septomaxilla - 2
Things that Go BMP in the Night: A Developmental Digression
It is at this point in phylospace that the septomaxilla begins to behave in a much more consistent and phylogenetically meaningful way. But, before describing this behavior, let us pause a moment to reflect on the implications of that last sentence. The ugly truth is that some morphological characters can be very useful phylogenetic indicators over certain ranges and completely misleading over others. Why should this be?
Development proceeds by a series of genetic cascade mechanisms. Suppose for example, that some high-level organizer is turned on, a Hox gene for example. In some cases, the Hox gene product is  exported and binds directly to receptor sites in another cell which cause that cell to differentiate into some particular definitive type. More often, the Hox gene product promotes the production and release of secondary signals. Which secondary signals are promoted depends on the developmental state of the receptor cell. These secondary signals then go out into the world and, again, either have direct effects or induce the production of tertiary signals -- and so on. Undifferentiated target cells probably receive a variety of conflicting signals. However, at some point, a threshold is reached and the cell becomes irreversibly committed to some particular histological fate.
exported and binds directly to receptor sites in another cell which cause that cell to differentiate into some particular definitive type. More often, the Hox gene product promotes the production and release of secondary signals. Which secondary signals are promoted depends on the developmental state of the receptor cell. These secondary signals then go out into the world and, again, either have direct effects or induce the production of tertiary signals -- and so on. Undifferentiated target cells probably receive a variety of conflicting signals. However, at some point, a threshold is reached and the cell becomes irreversibly committed to some particular histological fate.
These ultimate signals, carried by "bone morphogenic proteins" ("BMPs") and the like, launch sometimes complex developmental programs. However, the signal itself is simple, and the programs are rather stereotyped. One "fast twitch" muscle fiber is pretty much like another, everywhere in the body. While turning an undistinguished group of mesenchymal cells into muscle is a complex transformation, that transformation can be turned on or off with a set of very simple commands. What's more, these commands are part of the developmental vocabulary of mesenchyme cells generally, and are understood in more or less the same way throughout the embryo.
In the later Ottoman Empire, the first official act of the a new Sultan was normally to order the execution of any male siblings and, just to be on the safe side, selected first cousins as well. Embryos have a clear understanding of this salutary principle. There can only be one Sultan, one left jugal, one right coracoid, and so on. Literally the first thing a developing structure must do is to suppress the development of any other primordia which might show any ambition to become a jugal, coracoid, or, for that matter, a Sultan. Thus, there are a variety of negative signals as well, including signals instructing cells to die.
All embryonic cells, particularly in an area as complex as the vertebrate head, are subject to a variety of these signals. Which signal(s) ultimately activate differentiation depends on timing, geometry, and what the cell's neighbors are doing as well as the concentration of the inducers and repressors. However, clear differentiation is still the rule. A cell which is half muscle and half bone is useless for either function. Relatively sharp boundaries can be maintained because (a) the final developmental programs are mutually exclusive and (b) the Sultan Effect discussed above. Thus, we shouldn't be too surprised if a developing ossification center, such as the septomaxilla, has a discontinuous distribution of forms. Depending on small details of development, it may get sucked into the developmental pattern of low-level inducers generated by the cells shaping the nasolacrimal duct, the nasals, or the vomeronasal organ. However, its likely to be one form or the other -- not something in between.
For a small bone located at the intersection of several different developmental domains, the result is phylogenetic variability. The septomaxilla may be a good character for mapping temnospondyl families, for example, but its useless, and possibly misleading for broader scale studies. It can't tell us much about the relative position of temnospondyls and frogs, because it changes too fast and too often. The problem is a form of "long branch attraction" and exactly comparable to the problem of saturation in molecular studies. See discussion elsewhere.
But, what happens if the cells of the septomaxilla later become receptive to higher level inducers? Now, instead of responding to the simple messages, it responds first to high-level inducers like pax and hox. The state of the bone stops changing with every new family. We may observe a whole new set of smaller morphological changes as synapomorphies at the family level. Among the temnospondyls such slight changes would be ignored as phylogenetic noise -- at most apomorphies at the species level. These are now significant for higher order clades, while the bigger changes, such as the Types described above -- lose utility because they are essentially invariant.
Unfortunately, we can't expect that this sort of modal change will occur only at the borders of the phylospace of interest. So, together with all the other homology problems that plague cladistic analysis, we have to be concerned with whether we have this sort of problem as well. The typical parsimony analysis treats all changes as equal and all states with the same character score as homologous. How good are those assumptions in real life?
 Amniotes: Back to the Script
Amniotes: Back to the Script
With these vague and imponderable concerns behind us, we may return to the main line of our story refreshed and unburdened of all such feckless speculation. The only actual relevance of the foregoing osteological idyll is that the character of the septomaxilla does indeed change among the amniotes. No longer a volatile child of sudden, simple passions, it becomes a steady, but sophisticated, bone with a variety of intriguing specialized functions -- or disappears entirely.
Notwithstanding our concerns about Limnoscelis, the anapsid septomaxilla does appear to begin as a Type A morph as exemplified by Procolophon on the previous page. The bone is located on the posterior margin of the naris and includes a posterior process with significant external exposure. However, this is one of the few cases in which the typology is not completely clear, since the Procolophon septomaxilla also has an anterior process extending into the naris. This process looks suspiciously like the dorsal process of a Type B septomaxilla which has been rotated 90 degrees counterclockwise. Furthermore, the lacrimal has retreated from the naris in Procolophon, and the septomaxilla comes nowhere near it. It is, once again, hard to know how seriously to take detailed reconstructions of small, fragile elements such as the septomaxilla. However, something quite similar seems to be going on in some millerettids, in Bolosaurus, whose lacrimal does reach the naris, as well as in Acleistorhinus, where it doesn't.
In short, there seems to be the beginnings of a synapomorphic anapsid condition of the septomaxilla. Unfortunately, the whole thing peters out pretty quickly. In lanthanosuchids and pareiasaurs, the septomaxilla is strongly reduced or absent; and no turtle is known to possess one. There is nothing mysterious about this. The anapsids start out with elongate jaws and rostra, but the entire muzzle becomes progressively shorter across their phylospace. Perhaps this is a consequence of the reorganization of the jaw muscles towards the peculiar condition in turtles, in which the force of the adductor contraction is redirected over a "pulley" on the braincase or the pterygoid. Thus the force on the lower jaw is almost vertical. This gives great strength and efficiency to the jaw, eliminating the advantage (particularly in herbivores) and utility of a long tooth row.
The septomaxilla does not seem to have been much of a success among early Diapsida. The best known basal diapsids were aquatic, and the region around the nares was frequently unossified. Youngina, for example, seems to have had a septomaxilla, but its shape and position are unclear in Carroll's 1981) discussion. As we have mentioned, there is some correlation between the development of the septomaxilla and terrestriality. It would seem that this applies in reverse, as well. We have seen no report of a septomaxilla in any ichthyosaur or sauropterygian.
 Wherever the diapsids were hiding the genetic potential for developing a septomaxilla, it must have remained, since both rhynchocephalians and squamates have them. The bone is entirely internal to the naris and apparently tends to become involved in forming the nasal septum. However, we do not feel safe in referring to it as Type B. Not only is it relatively large and plate-like, but it immediately begins to behave in a rather peculiar fashion among the squamates.
Wherever the diapsids were hiding the genetic potential for developing a septomaxilla, it must have remained, since both rhynchocephalians and squamates have them. The bone is entirely internal to the naris and apparently tends to become involved in forming the nasal septum. However, we do not feel safe in referring to it as Type B. Not only is it relatively large and plate-like, but it immediately begins to behave in a rather peculiar fashion among the squamates.
For example, in the Phrynosomatidae, a family of desert lizards, the septomaxilla is posteriorly elongate and forms a "sink trap" for particulates entering the nares during subsurface burrowing. This is a U-shaped passage in which fine particles settle, allowing air to continue on freed of most contamination. When the lizard resurfaces, it expels the particulates with a violent sneeze. In Xenosaurus, the posterior septomaxilla penetrates deeply to participate in the choana. Wu & Huang (1986).
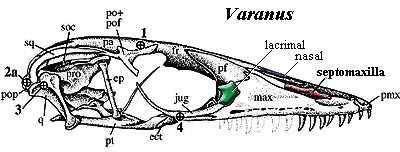 In snakes, this posterior process of the septomaxilla becomes ever more elaborate. As we have learned to expect, the septomaxilla is not known in the aquatic mosasaurs. However, even the very basal scolecophidian snakes have large septomaxillae. These are in extensive contact with the premaxilla anteriorly and the nasals medially. Ascending, medial processes of the premaxillae also lock this element between the nasals, so that the whole complex is tightly bound and interlocked, consistent with an important role in burrowing. The septomaxilla also bears a broad posterior process which clasps the vomers. Together with the vomers, the septomaxilla forms the floor of the nasal cavity and encloses the vomeronasal organ. Although a lacrimal is absent, the prefrontal bears the nasolacrimal duct which continues onto the septomaxilla. Romer 1956). The functional significance of this unique system is that it frees other palatal elements, particularly the pterygoids and maxillae, to move independently. In effect, the septomaxilla replaces both the lacrimal and the usual palatal bones as links between the mouth and the braincase. [3]
In snakes, this posterior process of the septomaxilla becomes ever more elaborate. As we have learned to expect, the septomaxilla is not known in the aquatic mosasaurs. However, even the very basal scolecophidian snakes have large septomaxillae. These are in extensive contact with the premaxilla anteriorly and the nasals medially. Ascending, medial processes of the premaxillae also lock this element between the nasals, so that the whole complex is tightly bound and interlocked, consistent with an important role in burrowing. The septomaxilla also bears a broad posterior process which clasps the vomers. Together with the vomers, the septomaxilla forms the floor of the nasal cavity and encloses the vomeronasal organ. Although a lacrimal is absent, the prefrontal bears the nasolacrimal duct which continues onto the septomaxilla. Romer 1956). The functional significance of this unique system is that it frees other palatal elements, particularly the pterygoids and maxillae, to move independently. In effect, the septomaxilla replaces both the lacrimal and the usual palatal bones as links between the mouth and the braincase. [3]
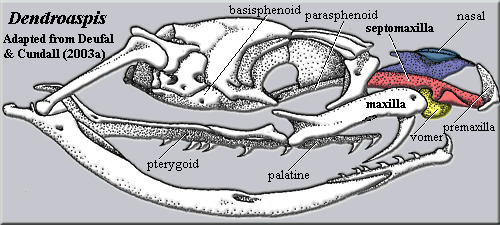 As the jaws become progressively loosened in more derived snakes, the septomaxilla-vomer complex undergoes a peculiar transformation from an incidental component of the nasal cavity to a major structural support. Thus, in aniloid snakes the septomaxilla - prevomer complex is still tightly bound to the palate by stiff ligaments. This stabilizes the upper jaw, but requires translational movement of each side of the entire complex (vomer + septomaxilla + palate) to "walk" the mouth over large prey items. Kley (2001). In colubroids, such as Dendroaspis pictured at right, the transformation is essentially complete. The main strut of the anterior skull is the septomaxilla, and its posterior process binds directly to the parasphenoid to brace the snout on the braincase. The pterygoid and palatine are unconstrained and able to move independently, while the maxilla rotates on the round vomer which serves as a sort of ball joint. Deufel & Cundall 2003); Deufel & Cundall 2003a).
As the jaws become progressively loosened in more derived snakes, the septomaxilla-vomer complex undergoes a peculiar transformation from an incidental component of the nasal cavity to a major structural support. Thus, in aniloid snakes the septomaxilla - prevomer complex is still tightly bound to the palate by stiff ligaments. This stabilizes the upper jaw, but requires translational movement of each side of the entire complex (vomer + septomaxilla + palate) to "walk" the mouth over large prey items. Kley (2001). In colubroids, such as Dendroaspis pictured at right, the transformation is essentially complete. The main strut of the anterior skull is the septomaxilla, and its posterior process binds directly to the parasphenoid to brace the snout on the braincase. The pterygoid and palatine are unconstrained and able to move independently, while the maxilla rotates on the round vomer which serves as a sort of ball joint. Deufel & Cundall 2003); Deufel & Cundall 2003a).
A septomaxilla "is an element that has never been reliably identified in any crowngroup archosaur." Hill et al. (2003). In fact, we are not aware of a septomaxilla in any archosauromorph. However, septomaxillae continue to be identified -- unreliably, one presumes -- in all kinds of archosaurs: phytosaurs, ankylosaurs, and confuciusornithid birds. For the most part, this proves only that a small snout bone is easy to misidentify.
CONTINUED ON NEXT PAGE
checked ATW050914
 exported and binds directly to receptor sites in another cell which cause that cell to differentiate into some particular definitive type. More often, the Hox gene product promotes the production and release of secondary signals. Which secondary signals are promoted depends on the developmental state of the receptor cell. These secondary signals then go out into the world and, again, either have direct effects or induce the production of tertiary signals -- and so on. Undifferentiated target cells probably receive a variety of conflicting signals. However, at some point, a threshold is reached and the cell becomes irreversibly committed to some particular histological fate.
exported and binds directly to receptor sites in another cell which cause that cell to differentiate into some particular definitive type. More often, the Hox gene product promotes the production and release of secondary signals. Which secondary signals are promoted depends on the developmental state of the receptor cell. These secondary signals then go out into the world and, again, either have direct effects or induce the production of tertiary signals -- and so on. Undifferentiated target cells probably receive a variety of conflicting signals. However, at some point, a threshold is reached and the cell becomes irreversibly committed to some particular histological fate. Amniotes: Back to the Script
Amniotes: Back to the Script
 In snakes, this posterior process of the septomaxilla becomes ever more elaborate. As we have learned to expect, the septomaxilla is not known in the aquatic
In snakes, this posterior process of the septomaxilla becomes ever more elaborate. As we have learned to expect, the septomaxilla is not known in the aquatic  As the jaws become progressively loosened in more derived snakes, the septomaxilla-vomer complex undergoes a peculiar transformation from an incidental component of the nasal cavity to a major structural support. Thus, in
As the jaws become progressively loosened in more derived snakes, the septomaxilla-vomer complex undergoes a peculiar transformation from an incidental component of the nasal cavity to a major structural support. Thus, in