 II. The Sensory Units
II. The Sensory Units| Bones: The Ear | ||
| The Vertebrates | Ear Overview (1) |
| s ("Cladograms") |
||||||
| Vertebrates Home | Vertebrate | Vertebrate | Bones | Time |
| Ear Overview The Incus References |
Why, you may be asking, is there a section on hearing in Bones? In part, it is here because there was no other reasonable place to put it. However, the choice is not entirely random. Many of the more confusing, difficult and significant osteological debates in paleontology and evolution have surrounded the collection of mechanoreceptors concentrated in the ear. For those who have been through Carroll (1988) or some equivalent text, consider how much debate and effort has gone into issues such as (a) the number and shape of the semicircular canals in jawless fishes, (b) the presence or absence of an otic notch in stem tetrapods, (c) the embayment of the quadrate or squamosal to support a tympanic membrane in various amniote lineages, (d) the shape, mass and orientation of the stapes as a sound conductor, or (e) the transformation of the post-dentary jaw bones into auditory ossicles in early mammals. All of these issues are significantly related to the ear and its sensory functions. Therefore, it makes some sense to digress into the anatomy and physiology of the ear as a phylogenetic and functional unit.
With our customary functional and phylogenetic approach, we will begin with a brief overview of the functions of the ear. After this we will approach the subject from the inside out, beginning with the oldest and most basic functional units of mechanoreception in the inner ear and moving on to progressively higher levels of organization and more recent evolutionary developments.
Some text writers (as well as earlier portions of these Notes) have persisted in the naive description of the ear as the sensory home of "hearing.". In fact, the ear is unique in being the center of at least three senses, each associated with a different set of physically separate receptors: (1) hearing, 2) angular acceleration, and (3) gravitational orientation (or linear acceleration). Probably there is at least one more "sense" involving 4) detection of low frequency vibrations, but it seems to overlap with the gravitational sense in the macular receptors (explained below). Much of the detailed physiology is poorly understood, even today. Furthermore, it appears that these senses do not merely supply information, but also elicit hard-wired reflex responses without going through the brain at all.
 II. The Sensory Units
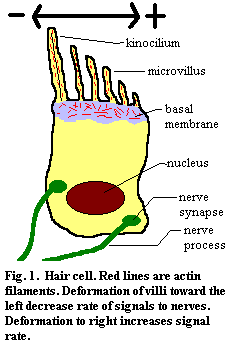
II. The Sensory UnitsAll of the senses in the ear, as well as the lateral line system of fish, are based on mechanosensation: the detection of physical displacement. The hair cell is the basic cellular unit of mechanosensation. It is characterized by the presence of oriented microvilli and a single kinocilium. The microvilli are stiffened by the presence of numerous oriented contractile actin fibers and are embedded in a basement membrane which includes randomly-oriented actin. When the microvilli are deformed by mechanical pressure or vibration, the cell increases or decreases the rate at which it sends an electrical signal to the nerve cells with which it is coupled. The "stiffness" of the microvilli, and hence their degree of response to a deforming force, can be regulated by the contractile state of the actin filaments.
Frequently, hair cells are highly directional. If the villi are bent on one direction, the rate of discharge increases. In the opposite direction, the rate decreases. If the villi are deformed at right angles to this axis (into or out of your screen in Figure 1), there is no change in the signal rate.
 In living organisms, the hair cells are organized into neuromast organs. The neuromast organ consists of hair cells and support cells embedded in a gelatinous cupula. The hair cells within the neuromast are sometimes oriented in opposite directions, but in only in those two directions. If Figure 2 represented such a case, no hair cells would be oriented into or out of the plane of the screen.
In living organisms, the hair cells are organized into neuromast organs. The neuromast organ consists of hair cells and support cells embedded in a gelatinous cupula. The hair cells within the neuromast are sometimes oriented in opposite directions, but in only in those two directions. If Figure 2 represented such a case, no hair cells would be oriented into or out of the plane of the screen.
The cupula, particularly in the macular, "gravitational" receptors, may bear otoconia, also known as otoliths. These are tiny [1] mineral grains, usually calcium carbonate. The otoliths supply the mass necessary to deform the entire cupula in response to linear acceleration or orientation in a gravity field. Some fishes use mineral grains from the environment for this purpose, and a few taxa manufacture calcium phosphate otoliths.
 The inner ear is located a cavity formed by the prootic or equivalent portion of the braincase in close association with the otic region of the brain and the VIIIth cranial nerve. It contains the vestibular apparatus which is shown, in extremely generalized form, in Figure 3. The vestibular apparatus us suspended by ligaments to isolate it from random vibrations in the skull. The vestibular apparatus is geometrically quite variable. However, it can be broken down into functionally constant regions, if at the risk of some over-simplification.
The inner ear is located a cavity formed by the prootic or equivalent portion of the braincase in close association with the otic region of the brain and the VIIIth cranial nerve. It contains the vestibular apparatus which is shown, in extremely generalized form, in Figure 3. The vestibular apparatus us suspended by ligaments to isolate it from random vibrations in the skull. The vestibular apparatus is geometrically quite variable. However, it can be broken down into functionally constant regions, if at the risk of some over-simplification.
The vestibular apparatus must have some method of equalizing pressure, or it would explode during changes in pressure. I am therefore a bit skeptical when it is described as a closed system. However, this style of pressure regulation is particularly important in aquatic vertebrates, since they must respond to rapid changes in external pressure with water depth. So, for example, in elasmobranchs, the inner ear is explicitly connected to the environment via the endolymphatic duct.
The labyrinth is composed of the semicircular canals (SCCs) and associated ampullae In almost all vertebrates, there are three SCCs (but see, e.g., Ateleaspis), each oriented roughly 90 degrees from the other two, such that they correspond to the three spatial planes. The SCCs themselves have no sensory role. They are simply fluid-filled structures. Their importance lies in how they slosh.
Imagine that Figure 3 is quickly rotated clockwise. For the most part, there will be no net movement of the fluid in two of the SCCs (the horizontal canal and the canal directed into the screen) relative to the walls of these canals. However, in the SCC facing to the right, the inertia of the fluid will cause it to flow counter-clockwise relative to the walls of this canal.
This current will move through the ampulla at the bottom of the SCC. Each ampula contains a crista, a very large neuromast organ, which stretches across the ampulla. When the fluid in the SCC flows, it creates a shearing force on the cupula. The hair cells communicate this movement to the brain. Thus, the ampulary receptors are sensitive to rotation or, more generally, angular acceleration. Because of the geometry of the SCCs, the deformation signals in the three ampulae can be combined to determine the rate and direction of rotation.
The maculae consist of rather shapeless compartments known as the sacculus and utriculus. elasmobranchs and perhaps other groups posses a third macula, the macula neglecta. The macular neuromast organs bear otoliths, as described above. Thus, they are sensitive to orientation in a gravity field or, more generally, to linear acceleration. The neuromasts in the sacculus are apparently oriented oppositely from those in the utriculus. However the geometry of these spaces is sufficiently complex that there is no simple correspondence between the macula and any particular orientation, at least in humans.
In addition, the macular receptors are involved in the perception of low frequency sound. Exactly how this works seems to be unsettled. The macular receptors are the primary organ of hearing in fishes, where the inner ear may be coupled to vibrations in the medium through a variety of mechanisms discussed below. In amphibians the inner ear is mechanically coupled to the pectoral girdle through the operculum. This mechanical coupling results in a "seismic" sense, which detects low-frequency vibrations in the substrate, such as those caused by platoons of loutish undergraduates dragged out on field trips to collect amphibians. Even in humans, at least some macular hair cells are involved in the perception of loud, low-frequency sounds. These inputs may, according to one recent story, have deep psychological effects, presumably because macular inputs can completely bypass higher brain processing. Think, for example, of the effects of thunder, marching, resonant choral singing, of singing alone (which is internally very loud and resonates in the skull), or of a roaring or growling animal. Sounds which are deep enough or loud enough to literally rattle your bones are generally perceived differently from normal sounds and often have irrationally strong psychological impacts. This appears to be tied to the unusual wiring of this ancient, auxiliary sense provided by the macular receptors, which may bypass the higher processing centers of the brain.
The lagena, as a structure, is almost as ancient as the labyrinth. However, it appears as just another macular compartment in fish and early tetrapods. An elongated distal process of the lagena is a common character of all of the amniote lineages, so we may suppose that the development of this structure predated, or was coincident with, the amniote divergence. In mammals, the lagena is coiled and is referred to as the cochlea. The lagena is the site of hearing, as that term is generally understood.
 The physiological details of the auditory sense are somewhat beyond the scope of this essay. However, since the original version of this page was written, we have learned that some aspects of this exceedingly technical subject are germane to paleontology. This horrifying discovery was revealed to us through the work of Dr. Zhe-Xi Luo. See, e.g. Luo & Eastman (1995). We are thus presented with an acute moral dilemma. On the one hand, Dr. Luo's work is important. On the other hand, this stuff takes anatomical obscurity to a whole new level. Now, the astute (or simply cynical) reader may already have observed that moral considerations rarely seem to slow us down much. True. But Dr. Luo has also said some very kind things about this site, and flattery is hard to come by. Accordingly, we will -- not for the first time, to be sure -- allow the dictates of our bloated ego to overcome our better pedagogical judgment and so attempt a brief explanation.
The physiological details of the auditory sense are somewhat beyond the scope of this essay. However, since the original version of this page was written, we have learned that some aspects of this exceedingly technical subject are germane to paleontology. This horrifying discovery was revealed to us through the work of Dr. Zhe-Xi Luo. See, e.g. Luo & Eastman (1995). We are thus presented with an acute moral dilemma. On the one hand, Dr. Luo's work is important. On the other hand, this stuff takes anatomical obscurity to a whole new level. Now, the astute (or simply cynical) reader may already have observed that moral considerations rarely seem to slow us down much. True. But Dr. Luo has also said some very kind things about this site, and flattery is hard to come by. Accordingly, we will -- not for the first time, to be sure -- allow the dictates of our bloated ego to overcome our better pedagogical judgment and so attempt a brief explanation.
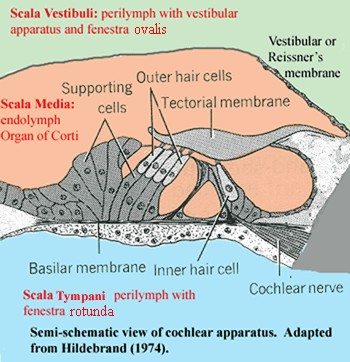
The illustrations in this section show the vestibular apparatus (the structures in Figure 3, taken as a whole) as if they simply floated in the perilymph of the inner ear. For the bulk of the vestibular apparatus, that is approximately true, although the general orientation is maintained by loose ligamentous connections. However, around the lagena (or cochlea) things are a bit more organized. Here the soft tissue lining the inner ear narrows to form a vessel, the perilymphatic duct. The perilymphatic duct runs along one side of the lagena for its entire length, from proximal (near the rest of the vestibular apparatus) to distal (away from the vestibule).
Looking only at this region, we may consider the inner ear to consist of two elongate, fluid compartments, running side by side. The image shows a cross-section of the structure. Ignore the blue area for the moment, and imagine the orange and green areas as tubes which run into and out of the screen. The green area is the perilymphatic duct. Since it is confluent with the perilymph around the rest of the vestibular apparatus, this compartment is called the scala vestibuli. The orange area is the endolymph-filled body of the lagena. It is referred to as the scala media. These two compartments are separated only by a thin, flexible membrane: the vestibular or Reissner's membrane.
Inside the lagena, another membrane runs the length of the scala media. One side of this membrane is anchored on a lateral wall of the lagena, but the other side floats more or less freely in the middle of the lagenar tube. This is the tectorial membrane. The free side of the tectorial membrane is in contact with the microvilli of a column of hair cells, very much the same as the hair cells in a lateral line system. These hair cells are mounted on supporting cells, and eventually anchored in a basilar membrane on the side of the lagena opposite the perilymphatic duct. For our purposes, we may consider the basilar membrane as a fixed, rigid, and inflexible platform. This is nearly correct, particularly in reptiles.
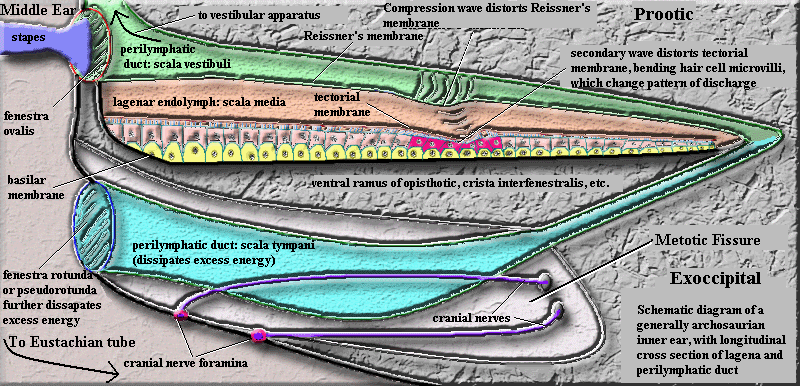
So much for structure. How does it all work? Know that matters are so arranged that the base of the perilymphatic duct is the part of the inner ear sitting on top of the fenestra ovalis. We will discuss this structure in excruciating detail below. Briefly, the fenestra ovalis is the small membrane "window" between the inner and middle ears. Sound is conducted through the middle ear via the stapes or columella (both are ear ossicles derived from the hyomandibular). The stapes has a footplate which fits over the fenestra ovalis. Sound vibrations in the stapes thus cause the footplate to rattle the window of the fenestra ovalis. Because the open end of the perilymphatic duct is sitting on the fenestra ovalis, each jerk of the membrane in the fenestra causes a compression wave to go shooting down the length of the scala vestibuli, which is side-by-side with the thin Reissner's membrane of the lagena. As each pulse moves along the perilymphatic duct, it pushes on the thin fabric of Reissner's membrane. Like running a finger along the side of a water balloon, this impulse generates a complex wave in the lagena. The internal wave in the lagena causes the free end of the tectorial membrane to wobble. Since the tectorial membrane is connected to the thin microvilli of hair cells which are essentially fixed, the microvilli experience shear -- exactly as in a lateral line neuromast, and with the same result.
The linear extent of the system also allows pitch discrimination. Exactly how this works is still not completely understood and may differ somewhat among lizards, crocs, dinosaurs, and mammals, each of which independently evolved this system [2]. Relatively recently, it has been learned that the hair cell response is not the signal which is passed directly to the brain as sound in mammals. Rather, the outer hair cells control a complex and poorly-understood system of positive and negative mechanical feedback systems, the net effect of which is to sort incident vibrations by frequency. Hair cells near the base of the cochlea respond to high-frequency sounds, while cells near the end of the cochlea respond to low frequencies. Outer hair cells which respond to the appropriate frequency reduce the propagation velocity of the membrane vibration to zero and maximize its amplitude. In effect, they trade the translational kinetic energy of a traveling wave for more displacement energy (i.e. amplitude) in a standing wave. It is this coordinated, high-amplitude displacement which registers on the inner hair cells, and the inner hair cells tell the brain about sound of a particular frequency.
continued on next page
last modified ATW060131
checked ATW050911