 c. Gnathostomes
c. Gnathostomes| Bones: The Gill Arches | ||
| The Vertebrates | Meckel's Cartilage |
| s ("Cladograms") |
||||||
| Vertebrates Home | Vertebrate | Vertebrate | Bones | Time |
| Bones
|
Gill Arches
Overview |
Meckel's Cartilage, also known as the Meckelian Cartilage (hereinafter the "MC") is best known as the original and most primitive lower jaw. However, it is quite different from anything previously attempted in Bones, and not just because it has a funny name. Meckel's cartilage is not one of those arriviste dermal bones that had no family history prior to Cheirolepis. It is not even a bourgeois endochondral braincase element dating to the origins of Gnathostomata. The MC dates back before the gnathostomes: quite possibly into early chordate and Precambrian times. Further, it lies at the heart of one of the truly large questions of paleontology: the origin of jaws. It has been said (if only because I said it myself, just now) that the history of the vertebrates is the history of jaws. Efficiencies in intake drove increases in mobility and metabolism in vertebrates. Further efficiencies and specializations drove further evolutionary changes in the skull and spread vertebrates over a huge range of environments. We may not be what we eat; but, most certainly, we are how we eat.
The anatomy of the MC is not worth a great deal of attention. Its derivatives get somewhat complex. These include the malleus, the articular, and the amphibian mental bone, as well as some ligaments and soft tissues. But these will be dealt with under their own names in later sections. The MC itself is merely a cylindrical piece of gristle without much character or flavor. The phylogeny is what we will focus on. As a result, the history of the MC will be taken very slowly, with attention to a phylogenetic story that is lengthy, but still very incomplete. We will then review some of what is known about the embryology of the MC and its developmental molecular biology. Finally, we will briefly reassess the evolutionary story in light of this information.
As noted in the gill arch overview, the MC is supposedly the "ceratomandibular," the lower main element of a hypothetical primitive first gill arch, the "mandibular" arch. Current thinking is that the branchial apparatus was originally not designed for respiration. The early chordates were small and inactive enough to directly absorb oxygen over the epidermis generally. Indeed some sizeable turtles and lissamphibians are able to get by in this fashion even today. Organisms along the lines of the cephalochordate amphioxus use the gill apparatus for filter feeding. The branchial slits, in this context, are simply vents to release excess water which has been filtered for nutrients. Already, in this group, the branchial apparatus is supported by a series of external pharyngeal bars. As we will see, these are analogous, but not homologous, to gill arches. Anterior to the "gills," amphioxus has a velum, but it is merely a passive regulator of intake which works a bit like the iris of a camera. The flow of water is actually provided by a battery of microscopic cilia.
Amphioxus is a virtually sessile filter feeder. Basal craniates run much larger than amphioxus and appear to be designed for a higher degree of energetically expensive mobility. To judge from hagfish and lampreys, the only living jawless fish, the basal craniates had also taken up moderately taxing hobbies, such as parasitism and scavenging dead or weakened fellow craniates.[1] In order to support these disgusting habits, some taxa developed adaptations for sucking in both food and respiratory water. The most common respiratory pump was an adaptation of the velum. Among craniates, it became an active pump rather than a regulator. The detailed anatomy and workings of the velum are not yet tackled here. Suffice to say that the velum is a sail-like curtain of tissue (hence the name) which pushes water into the pharyngeal passage and is supported and controlled by a velar skeleton. Significantly, the velum lies immediately adjacent to the otic capsule.
By the level of the Osteostraci and lampreys, i.e. at least as early as the Silurian, a typical large fish might have a velum supported by a velar skeleton towards the center of the branchial passage, and several respiratory gills, externally reinforced by an interwoven "branchial basket" of bone or cartilage braced against the body wall and connecting the gill pores, like a chicken wire fence. In addition, Mallatt (1996) has argued, based on a detailed analysis of the ammocete larvae (see image) of lampreys, that an internal pharyngeal skeleton was also present. Although lost in adult lampreys, the ammocete larva seems to have both internal and external pharyngeal supports.
Mallatt argues that such internal arches were the rule, rather than the exception, despite the lack of specific fossil evidence. Thus, for example, he argues that such elements were present in osteostraci, but failed to fossilize. This is perhaps a bit hard to swallow, so to speak, because the incredibly well-preserved remains of many osteostracans from Spitzbergen and Estonia clearly show even the most delicate neural structures. Not only are internal gill arches not found, but one might well ask why the osteostracan gill would require any terminal support at all, inside or out, given that all of the gills were embedded in one enormous, solid block of head shield cartilage for their entire length.[2] Perhaps the jointed gill arches of the fossil lamprey relative Haikouichthys Chen et al. (1999)) provide better confirmation of the theory, but only if one is willing to interpret these structures as internal to the gills. In this connection, however, note that these elements are not very different from the undoubtedly external structures found in the Anaspida generally, particularly the triradiate bone which is the singular synapomorphy of the clade.
The alternative argument, advanced by Janvier 1996) is in fact not all that different. Janvier agrees that gills, and perhaps the external branchial skeleton, are primitive to the chordates. However, he sees the internal branchial skeleton as a neomorphic gnathostome character. To simplify, the matter comes down to the velum, which is agreed by all to be related in some manner to the mandibular arch. Mallatt views the velum as an accessory structure anterior to the mandibular arch which may or may not have been present in the ancestor of gnathostomes, but which disappeared in that line without a trace. However, the velum provided a major evolutionary (as well as hydrostatic!) pressure to stabilize the gills with an internal skeleton. By contrast, Janvier sees the velum as the first development and asserts that the internal branchial skeleton was developed by serial duplication and adaptation of the velar skeleton in early gnathostomes. To Mallatt, the velum is, at most, an evolutionary precondition of the branchial arch. To Janvier, it is a structural precondition.
 c. Gnathostomes
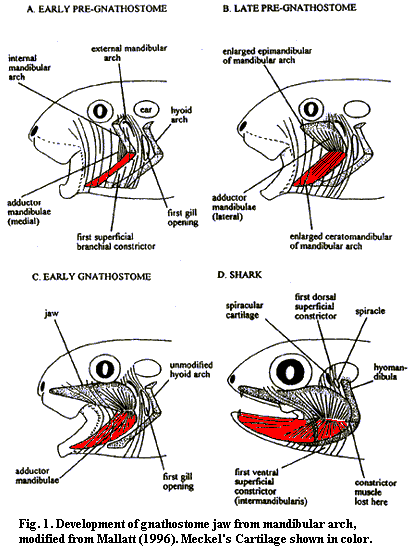
c. GnathostomesWe are now ready to sink our teeth into the core problem: the origin of jaws. Mallatt's transformation series is well known. Figure 1. In this view, the jaw is derived from an ordinary respiratory arch, with the lower jaw, the MC, being derived from the ceratomandibular. The embryos of some fish doing a very convincing imitation of this series during development. The external mandibular skeleton disappears altogether. Mallatt sees this transformation as driven by increased need for ventilation, and only later becoming a grasping jaw. During this sequence, the external branchial basket disappears, except for some remnant cartilage which is still found externally supporting the gills of chondrichthyans. During development, the mandibular and hyal arches are coopted into the jaw and the first gill opening is reduced to a small spiracle.
Janvier points out a number of problems with this evolutionary scenario. For example, no fossil form is known in which the first gill arch has a respiratory function, with the possible exception of very derived pteraspidomorphs, which are well off the gnathostome line. Mallatt reconstructs the osteostraci as having a respiratory mandibular arch. However, although gill impressions are known in this group, they do not occur at this anterior position. Thus Janvier reconstructs the osteostraci as having a velum, but of course there is no evidence of a velar skeleton either. Janvier also notes that the spiracle is not an outlet, like the gill slits, but an entrance. Further, the external gill skeleton of jawless fish is never known to reach as far forward as the supposed mandibular arch.
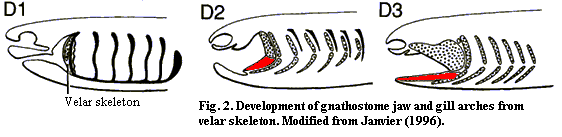
Janvier's transformation series is shown in Figure 2. In this version, both the gill arches and the jaws are serial homologues of the velar skeleton. Janvier posits that both developed from the velar skeleton at the same or similar times. However, there is nothing in his hypothesis which really demands this result.
Truthfully, it is impossible to make a firm decision based on anatomical grounds alone. In fact, it is impossible even to summarize the anatomical evidence in an even-handed manner within the body of an essay of this scope. Mallatt's theory has the virtue of elegance, and he may have the edge in anatomical evidence (except possibly neuroanatomy) from living species. Janvier's hypothesis has a corresponding advantage on the fossil record. One suspects, however, that these apparent differences may be related to the fact that Mallatt has simply spent more time cutting up fish while Janvier has spent more time staring at rocks.
 So, the question may now be framed: is the jaw a serial homology of the internal branchial arches, or were both jaw and branchial arches independently derived from a velar skeleton? Were we all Americans, we might now take a vote, after which a black-robed chorus of elderly lawyers, solicited at great expense, could tell us what it was we had decided. Fortunately, there may be marginally more satisfactory ways of arriving at the truth of this matter. The effort will require us to abandon the warm and familiar neighborhood of anatomy and venture out into some rather cold, serious and very new molecular biology. From that radically different perspective we will see that Janvier is probably correct. First, however, not to completely lose the thread of our story, we will briefly review the last 400 My of evolution of the MC.
So, the question may now be framed: is the jaw a serial homology of the internal branchial arches, or were both jaw and branchial arches independently derived from a velar skeleton? Were we all Americans, we might now take a vote, after which a black-robed chorus of elderly lawyers, solicited at great expense, could tell us what it was we had decided. Fortunately, there may be marginally more satisfactory ways of arriving at the truth of this matter. The effort will require us to abandon the warm and familiar neighborhood of anatomy and venture out into some rather cold, serious and very new molecular biology. From that radically different perspective we will see that Janvier is probably correct. First, however, not to completely lose the thread of our story, we will briefly review the last 400 My of evolution of the MC.
The gnathostomes are generally accepted as monophyletic, but the method by which the jaw is attached to everything else has been quite varied from the very beginnings. That is really part of the palatoquadrate story, and we will not review it here. In both placoderms and chondrichthyans the MC continued to serve as the primary lower jaw element directly. In this capacity, it came to bear a very wide variety of cutting surfaces, from the inferognathals of arthrodire placoderms to the bizarre spiral symphysial tooth whorls of Helicoprion. The very diversity of these forms of dentition is perhaps a strong argument that the MC first became a jaw element in the chondrichthyans, since other gnathostome clades have, by comparison, a rather limited repertoire of tooth forms.
In osteichthyans, the jaw adductors insert medially in the lower jaw, and the MC comes to be covered by dermal bones, including the dentary, angular, surangular and splenials. Although the MC remains a simple rod-like cartilage as a whole, the proximal end ossifies and becomes the articular bone which continues to form the lower jaw articulation (or one of the lower jaw articulations in the case of teleostomes and advanced therapsids) in virtually all groups except mammals.
The transition to land seems to have had almost no effect on the MC except that, in some lissamphibian groups, the distal end of the MC also may ossify as the mental bone. Despite numerous changes in dentition, the dermal bone covering, and various episodes of radical kinesis or equally radical skull consolidation, the MC has remained a small, but consistent element of the lower jaw. In some lepidosauromorphs, including sauropterygians and agamid lizards, the MC even makes a modest come-back as a superficial element on the inner (lingual) surface of the mandible.
This constancy in the very teeth (!) of obsolescence may relate to two factors. First, the MC may still provide some marginal advantage by providing those qualities that favor cartilage over bone: flexibility and elastic compressibility (i.e., the ability to act as a shock absorber). Probably more importantly, the MC is still the embryonic lower jaw. The correct positioning and shape of the adult mandible depends on the MC regardless of whether the MC has any functional significance for the adult. This may constitute another, and rather different, example of the rule suggested in our discussion of the premaxilla: an element which becomes involved in several different functional units acquires evolutionary stability. However, the stability of the MC requires us to ask a more refined question. Is this a rule enforced by physiology or by ontogeny? To put the matter another way, are the constraints which stabilize a bone most likely to be imposed by survival and reproduction of the adult, or by the effect it has on the development of other units? Arguably, we now have an example of each. The premaxilla is a relatively late developmental and evolutionary component which has no obvious ontological significance but has important adult functions in several disparate areas. The MC is a critical component in development, but probably has little functional importance in the adult.
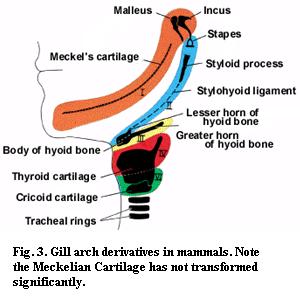 Again, I will attempt no answers. However, it may be significant that the functions of the adult MC can be changed more radically than the functions of the adult premaxilla. Thus, during mammalian development, the proximal region of the MC is transformed into the malleus (or the malleus and incus, depending on who you read), an ossicle in the mammalian middle ear. However, this is probably only a further transformation of the already transformed articular bone. The MC itself has remained resistant to any pressures that may exist for evolutionary change -- a notable stability considering the radical re-engineering of the mammalian jaw. This constancy is particularly interesting in view of the variety of transformations seen in the other mammalian gill arch derivatives. See Figure 3. This may be a hint that there is something a little special about the mandibular arch, or at least the MC. However, to get more than hints, we must turn to microscopes and molecules.
Again, I will attempt no answers. However, it may be significant that the functions of the adult MC can be changed more radically than the functions of the adult premaxilla. Thus, during mammalian development, the proximal region of the MC is transformed into the malleus (or the malleus and incus, depending on who you read), an ossicle in the mammalian middle ear. However, this is probably only a further transformation of the already transformed articular bone. The MC itself has remained resistant to any pressures that may exist for evolutionary change -- a notable stability considering the radical re-engineering of the mammalian jaw. This constancy is particularly interesting in view of the variety of transformations seen in the other mammalian gill arch derivatives. See Figure 3. This may be a hint that there is something a little special about the mandibular arch, or at least the MC. However, to get more than hints, we must turn to microscopes and molecules.
The best and most comprehensive source of WWW information on branchial arch development is at School of Anatomy - ANAT2310 Session 2 Lecture 2, including the notes at UNSW Embryo- Head and Neck Development 1. Although this is a medical site, we lack an equivalent site dedicated, for example, to shark embryology. So we must parasitize the medical profession for now. As it turns out, the human gill arches are not really all that atypical.
Humans have five gill arches, which are conveniently known as arches 1, 2, 3, 4, and 6. Arch 5 is omitted, presumably for neuroanatomical reasons. We will quickly gloss over this issue by pretending we had not noticed the numerical anomaly. Interestingly, arches 1 and 2, the mandibular and hyoid, develop well before the others. At this point, the gross anatomy appears as in Figure 4.
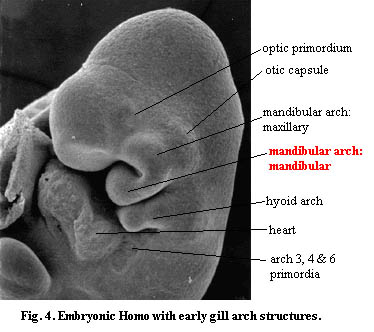 There are several features of interest. As mentioned, the mandibular and hyoid arches develop well ahead of the others. Note also the close relationship of the mandibular arch to the otic capsule. This is a typical feature. Although we have foresworn neuroanatomy, it is worth remembering that the mandibular arch is always enervated by the Vth cranial nerve. Even in osteostracans, where the gills are somewhat distant from the brain, the first arch (velum or gill, according to your preference) appears to be enervated by this nerve. The proximity to the otic capsule and this pattern of enervation are both found in the ammocete velum.[3].
There are several features of interest. As mentioned, the mandibular and hyoid arches develop well ahead of the others. Note also the close relationship of the mandibular arch to the otic capsule. This is a typical feature. Although we have foresworn neuroanatomy, it is worth remembering that the mandibular arch is always enervated by the Vth cranial nerve. Even in osteostracans, where the gills are somewhat distant from the brain, the first arch (velum or gill, according to your preference) appears to be enervated by this nerve. The proximity to the otic capsule and this pattern of enervation are both found in the ammocete velum.[3].
The branchial arches begin as cylindrical cores of mesenchyme sandwiched between continuous sheets of epidermal ectoderm and internal endoderm. The mesenchyme is then infiltrated by neural crest ectoderm migrating from the brain and the rhombomeres of the neural tube. (Some of these terms are explained a bit more at Early Development Notes.) The origin and targets of these agents are quite specific. Ectoderm from the mesencephalon and rhombomere 1 specifically migrates to the mandibular arch primordium, and rhombomere 4 infiltrates the hyoid arch. The remaining arches recruit variously from rhombomeres 7 and 8. Again, we see that the mandibular and hyoid arches are developmentally different from the rest of the series.
Recently, a number of molecular tools have been worked out which will allow us to look in more detail at these key events. At the moment, what is available is a series of very suggestive, but not yet decisive, experiments using the usual battery of knock-out mice, random Danio (zebrafish) mutants, ectopic addition of control factors and so on. Although the pathways are far from being worked out, all suggest that the mandibular arch contains the ground plan and that this plan is suppressed in the hyoid and subsequent arches, probably by homeobox genes, in favor of the gill arch plan. In particular, hox gene products are absent from the mandibular arch, but present in other arches. Hox genes specify the polarity and antero-posterior patterning of the vertebrate body and limbs, other than in some areas of the head. The pattern seems to be imposed on the various arches by the migrating neural crest ectoderm.
Thus, knockout hoxa-2 mutant mice exhibit (a) lack of mesenchymal neural crest cell derivatives in the hyoid arch; (b) change of second arch neural crest cell identity to first arch identity; (c) homeotic transformation of second to first arch skeletal elements, including: (i) duplication of ossification centers of bones of the middle ear and (ii) duplication of Meckel's cartilage adjacent to the otic capsule. Rijli et al. (1993); Gendron-Maguire et al. (1993). These workers attribute their result to reversion of the hyoid arch to a ground pattern established by mandibular arch patterning. Interestingly, a human mutant with similar, but less drastic, symptoms, has been described. Rodriguez et al. 1997).
By contrast, the posterior gill arches seem to be well-integrated into the post-cranial hox system. Specifically, these arches respond to hox gene products which are introduced from the neural crest ectoderm. Some of these wandering neural crest cells are merely tourists, passing through the region on their way to the heart, which lies at the posterior end of the gill arch series. In fact, the pericardial membrane is primitively co-extensive with the gill membranes.
A screen for point mutants in Danio revealed a class of mutation which affected only the first and second arches point mutants. Piotrowski et al. (1997). Curiously, these mutants showed deformations largely in the ceratal components, i.e. the MC and the hyomandibula. It is not yet clear what significance these mutants will have, although the Danio system is obviously an important one for our purposes.
More generally, experiments with various mouse systems suggest that MC differentiation is governed by a more primitive system based on epithelial-mesenchyme interactions involving gradient regulators such as shh, msx-1, msx-2, prx-1, prx-2, as well as growth factors of great generality such as EGF, BMP-2 and BMP-4. Barlow & Francis-West (1997); Lu et al. 1999); Shum et al. (1993); ten Berge et al. (1998). These regulatory systems are referred to as "primitive" because they appear to share at least some of the following characteristics: (a) a degree of dose-dependency, (b) partially redundancy (except for shh), (c) rather generalized effects in multiple areas of the embryo, (d) they, and their DNA targets, are scattered in the genome rather than being found in hox-boxes or similar arrangements, and (e) the regulatory pathways seem dependent on feedback loops between epithelium and mesenchyme. Time will tell whether "primitive" is a reasonable label. It is certainly a different system from the regulation of the posterior branchial arches. These have been thoroughly integrated into the post-cranial hox system.
Given this new information, we can tentatively conclude that Janvier has the better argument. We can, at least, say with confidence that there is no simple homology between the mandibular arch and the posterior gill arches. Obviously they share some common ancestral structure. However, the basal structure seems more likely to have been the velar skeleton than an internal gill arch. The posterior arches differ because (a) they are hox-regulated (b) appear later in development (c) are developmentally linked more closely with the heart as one might expect for gills) than the head [4]; and (d) at least the hyoid arch can be made to revert to the mandibular form by loss-of-function mutations. All things considered, this is a fairly strong set of indicators that the posterior arches are derived from a velar "arch," which has never had a respiratory function.
ATW 001126
[1] One wonders if the prevalence of this type of predation in the Silurian might not be the true reason for the nearly universal adoption of dermal armor among early gnathostomes, as well as its near universal abandonment by the Late Mesozoic.
[2] This may be unfair. Janvier 1996) is of the opinion (contra Stensiö) that the osteostracan gill apparatus, whatever it may have been, was "floating" freely in the head shield, rather than being integrated in the cartilaginous matrix. However this may be, mechanical support would not seem to have been a major evolutionary constraint inside this blockhouse skull.
[3] Hagfish have a homologous cranial nerve, including a "mandibular" component; but I have not been able to determine what it innervates.
[4] Recall that the heart is a relatively late craniate development. The hagfish has several "hearts" and the atrium and ventricle of the post-branchial pump are cleanly separated.
checked ATW030403