Neoselachii: Hypnosqualea
Taxa on This Page
- Batomorphii
- Hypnosqualea
- Narcinidae
- Pristidae
- Pristiophoridae
- Rajiformes
- Sclerorhynchidae X
- Squatinidae
- Torpedinidae
- Torpediniformes
Hypnosqualea: The Morph of the Batomorphs
Morphologically, the Hypnosqualea are just one more radiation of ray-like chondrichthyan fishes. Consider, by way of example, the Squatinactida, some of the Holocephali, or the Iniopterygii. Likewise, chondrichthyans with an extended rostral process -- like sawfishes -- are known from the batomorphs and the Bandringidae. A similar morphology is found in many members of the Chondrostei, who are not chondrichthyans at all, but also have a largely cartilaginous skeleton. In fact, both of these morphotypes, the ray-like and the sawfish-like, probably predate the Gnathostomata -- but that will be a subject for another day.
Of course, the Hypnosqualea are not just ray-like. They are the rays -- as well as the sawfishes and angel sharks. The Hypnosqualea were named and defined by Shirai in 1992. The "Hypnosqualea hypothesis," i.e. the unity of the rays, swordfishes and angel sharks, has been widely accepted. The current best-guess phylogeny is described in McEachran et al. 1996) and Shirai (1996). This tree has a topology along the following lines.
Protospinax may be the sister taxon of the Hypnosqualea. Maisey 1993). Since Protospinax is extinct, it's hard to be sure. The Hypnosqualea are more accurately anchored on the angel shark, Squatina, which is a sort of morphological intermediate between sharks and rays. The Pristiophoridae come next. These are sawfish-morphs, in overall appearance, but much closer to Squatina in their detailed anatomy. After this, things get a bit less clear. Either the Pristidae, the true sawfish, come next, or the electric rays of the Torpediniformes. We have chosen the latter course, following McEachran et al. 1996). In that case, Batomorphii (= Torpedo + Raja) is the node immediately below the pristiophorids. Branching off after these clades are Rhina and Rhinobatos, which are not yet covered in Palaeos as of this writing, and finally the conventional rays and skates. No one has a good handle on the Sclerorhynchidae, so we have arbitrarily placed them after the pristids.
With that out of the way, we can turn to the more interesting questions. What happened here? The Elasmobranchii had rolled along from the Early Devonian to about the Kimmeridgian Age of the Late Jurassic, over 250 million years, as very shark-like sharks. Then, over the next 40 or 50 million years (rather quickly by chondrichthyan standards), some of them went flat. We have speculated on the reasons for these events in the Overview. The issue we now address is, not so much why this occurred, but exactly what happened from an anatomical perspective.
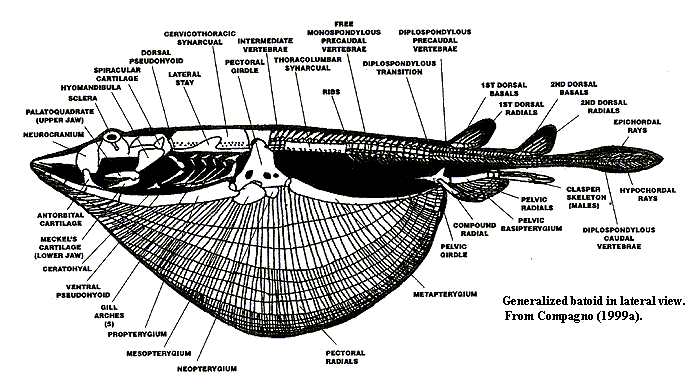 Starting from the front end, something significant happens to the rostrum. In the sawfishes, the rostrum is enlarged. It develops lateral "teeth" which may have sensory and/or attack functions. In this morph, the rostrum the rostrum encloses the precerebral fossa. By contrast, in rays, the rostrum is very short and broad. The precerebral fossa continues back, through the neurocranium, to a parietal fossa. If we accept that Hypnosqualea is a clade which has both ray and sawfish morphs at various points, we must accept that these two alternatives are somehow homologous. It is hard to see how this can be the case, but the overall unity of the Hypnosqualea is compelling. In both cases, separate ventrolateral and anterolateral cartilages on nasal capsules support the anterior of the head or attach to the propterygia. This may be the common denominator, as these unique cartilages may be oriented anteriorly, to support the rostrum of sawfishes, or laterally, to support the "wing" of rays. Labial cartilages are reduced or absent in all Hypnosqualea.
Starting from the front end, something significant happens to the rostrum. In the sawfishes, the rostrum is enlarged. It develops lateral "teeth" which may have sensory and/or attack functions. In this morph, the rostrum the rostrum encloses the precerebral fossa. By contrast, in rays, the rostrum is very short and broad. The precerebral fossa continues back, through the neurocranium, to a parietal fossa. If we accept that Hypnosqualea is a clade which has both ray and sawfish morphs at various points, we must accept that these two alternatives are somehow homologous. It is hard to see how this can be the case, but the overall unity of the Hypnosqualea is compelling. In both cases, separate ventrolateral and anterolateral cartilages on nasal capsules support the anterior of the head or attach to the propterygia. This may be the common denominator, as these unique cartilages may be oriented anteriorly, to support the rostrum of sawfishes, or laterally, to support the "wing" of rays. Labial cartilages are reduced or absent in all Hypnosqualea.
The eyes are moved dorsally and seem to undergo some simplification. The upper eyelid is lost, and the suborbital shelf is variably reduced. Many rays have reduced eyes, and a few are essentially blind.
The jaw is significantly re-engineered. The entire jaw apparatus is attached to the neurocranium only through the hyomandibular. There are no direct articulations with the "skull," and the ventral portions of the hyoid arch are lost. The entire quadrate process of the palatoquadrate is also lost, with real savings in mass. The jaw is a relatively small structure, essentially slung under the neurocranium on the hyomandibular, as well as the adductor musculature and several ligaments. The non-suspensory elements of the hyoid arch are taken over by neomorphs, the dorsal & ventral pseudohyals. These are formed from fused hyoid rays and articulate with the succeeding gill arches. As Shirai (1996: 21) notes:
... the feeding apparatus of batoids is totally different from that of sharks, not only in the presence or absence of the [orbital] articulation. The mandibular arch of sharks is supported by the whole hyoid arch posteromedially, and its protrusion is related to the rotation of the hyoid arch posteroventrally. ... In batoids, the hyomandibula directly suspends the whole mandibular arch and makes the jaws protrude by its swinglike motion ventrally.
The alteration of the hyoid arch is but the most anterior of numerous changes in the gill skeleton. The spiracles are enlarged, with more elaborate control by means of muscular valves. This has obvious functional significance, since the gill slits are ventral and may be blocked by substrate. The extrabranchial cartilages which often support the gill arches in sharks are reduced, but (possibly to address the same functional problem) may be partially recycled as "opercular" cartilage to strengthen the gill slits. Significantly, the last basibranchial articulates with the scapulocoracoid. We are uncertain whether there are any directly relevant in vivo studies, but it seems likely that breathing and locomotion are directly coordinated when the animal is "cruising."
The anterior spine is inflexible and rigid. All hypnosqualeans have some sort of cervicothoracic synarcual. Hypnosqualeans lack an occipital hemicentrum, but the synarcual is preceded by a suspiciously similar hemicentrum. Some, highly derived, rays have a second, thoracocolumnar synarcual behind the first synarcual.
The locomotor functions of the trunk and tail are supplemented, and ultimately replaced, by the pectoral fins. On the distal caudal vertebrae, the centra may be secondarily lost, leaving an unconstrained notochord. The tail is thus reduced, but the lack of locomotor function also frees the caudal region for other purposes. Thus, the tails of rays may develop barbs & stingers, with or without toxic secretions. Fin spines are lost from the unpaired fins. The first dorsal is moved back behind the pelvic girdle. The second dorsal may be far out on the tail, or even lost altogether.
As might be expected, the scapulocoracoid undergoes remarkable development. The scapular process is antero-posteriorly wide. A suprascapular cartilage is usual. Each suprascapular is attached to the neural arches of the spinal column and to its antimere. Primitively, this articulation is posterior to synarcual; but, in more derived forms, articulation is through the synarcual itself. An analogous development is seen on the ventral side, where the coracoid bar fuses at the midline. As mentioned above, the coracoid also articulates with terminal ceratobranchial through a coracoid condyle. All three basal axes undergo great elaboration. Each basal has a separate condylar articulation with the scapulocoracoid. The propterygium anterior basal of pectoral fin) comes in contact with an orbital process through an antorbital cartilage, and the pectorals are fused to side of head over external gill openings. In some forms, the principal articulations of the basals with the body are through these secondary contacts. In effect, these rays have multiple, independently moving pairs of arms. The pectoral fins themselves become plesodic. That is, the cartilaginous radials extend to margins of pectoral fin, functionally and physically displacing the keratinous ceratotrichia. The radials are highly branched distally and segmented.
The pelvic fins undergo a similar, but usually less drastic series of changes. The pelvic fins are also plesodic, with segmented, branched radials continuing to edge of fin. Curiously, in some rays, one lobe of the pelvic fin develops a structure very much like the tetrapod hind limb, which is used for substrate "walking." However, the details of this striking convergence will be dealt with later. In another tetrapod-like development, the rays lose almost all squamation, and the skin becomes smooth and glandular. ATW021019.
Torpediniformes: On Being Flat and Highly Charged
Among the Neoselachii, the Torpediniformes (electric rays) are perhaps the most extreme specialists. They form a fitting subject about which to ask questions about what it means for a vertebrate be flat. But see also, Abbott (1884). The electric rays of the torpediniform group are also perhaps the only reasonably diverse taxon in which essentially all members use electric organs as a means of predation. Since the electric organ gets all the press, the ray's overall predation strategy is not often described. Fortunately, Moller 1995) has summarized the hunting strategy of this peculiar group in a very readable form.
Most torpediniforms have relatively poor vision and some are effectively blind. Certain inshore species will actively hunt during the day, but the most common hunting strategy is ambush predation. In this mode, the ray covers itself with bottom sediment, leaving only its eyes and spiracle exposed. According to Moller, the rays do not use vision for hunting in this mode, but rely on pressure and electroreception alone. But, if this is the case, why are the eyes exposed and the lateral lines buried? Whatever the case, the rays only react to potential prey within a radius of about 10 cm. This suggests that rays must select their places of ambush with some care, since not every random spot on the ocean floor is likely to provide suitable prey animal encounters in that small a space. Perhaps the rays' diurnal cruising behavior represents a search for suitable hunting blinds, rather than food.
When prey does pass into the ray's reaction space, the fish rises up on the lateral edges of its pectoral fins and simultaneously flicks its tail so as to face the prospective meal. Given that torpediniforms are fond of relatively large, fast-moving fish, and that the ray is covered with sediment, this operation requires remarkable speed and strength. Speed is also necessary for prey capture, as the ray depends on the undercurrent created by its "jump" to suck the fish in under its mantle.
As the ray makes contact, the electric organs begin firing, generating potentials of up to 200V at frequencies up to 600 Hz. These are, of course, maximum values. More typically, a large specimen has been measured generating about 50V at 200 Hz. Not only is the voltage respectable, but the power output is close to 1kW over an attack sequence which may last as long as a minute. In prolonged struggles, the frequency, but apparently not the electrical potential, of the discharges gradually declines. The actual timing and strength of the electrical attack depends on the amount of resistance the prey puts up. The ray may also make secondary "jumps" to trap the fish. If successful, the ray envelops the victim in its broad pectoral fins and consumes it.
The electrical organs consist of modified branchial muscle tissues which are highly modified to serve as electrocytes, miniature organic batteries. An individual may have 200 to 1000 columns of electrocytes, each containing about 1000 individual cells. The electrocytes establish a charge difference across the cell membranes, presumably by active transport of ions. The discharge is controlled by signals sent through massively hypertrophied motor neurons coordinated by a medullary pacemaker, which cause the gradient to collapse coordinately across the posterior face of each electrocyte, resulting in a net surge of current through the cell column. The size of the organs and the frequency of the discharge raises an issue that I have not seen addressed. How is all this coordinated? Typical peripheral nerve conduction velocities in humans are around 55 m/sec. If this is even approximately similar to the figure applicable to ray electrical organs, then the time difference between the signal reaching the most proximal and most distal parts of the electrical organs may be a significant fraction of the discharge duration (i.e. 2-5 msec). It appears to be important that all of the electrocytes fire as simultaneously as possible. If these assumptions are correct, there must be some mechanism to coordinate the timing so that the closer cells do not fire appreciably before the more distal cells. ATW000618
Descriptions
Hypnosqualea: defined as Squatina + Raja.
Range: from the Early Jurassic
Phylogeny: Squalea:: Protospinax + *: Squatinidae + (Pristiophoridae + Batomorphii).
Characters: $ ethmoidal canal present with exit on dorsal surface of nasal capsules [dC96, 5(2)] [S96, 6(2)]; $ subnasal fenestrae absent (reversal) [S96, 7(0)]; $ basioccipital fovea present [dC96, 16(1)] [S96, 20(1)]; $ lower jaw teeth arranged diagonally [S96,
-96(0)]; $ m. inclinator dorsalis originating from vertebrae not epaxial musculature) [dC96, 52(1)] [S96, 88(1)]; $? ventral caudal muscle bundle well-developed [dC96, 53(1)]; $ m. flexor caudalis extends anterior to origin of caudal fin [dC96, 54(2)] [S96, 90(2)]; $? separate articular condyle for pectoral metapterygium present [dC96, 38(1)]; $ pectoral propterygium extended anteriorly [?dC96, 41(1)] [S96, 59(1)].
Links: The American Elasmobranch Society Hompage Carvalho & Maisey abstract).
References: deCarvalho 1996) [dC96]; Kemp (1999) [K99]; Shirai (1996) [S96]. ATW030116.
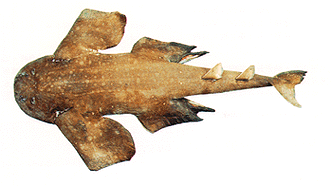 Squatinidae: angel sharks. Pseudorhina, Squatina
Squatinidae: angel sharks. Pseudorhina, Squatina
Range: from the Late Jurassic, currently Atlantic, Pacific & Mediterranean. Squatina known from Late Cretaceous.
Phylogeny: Hypnosqualea: (Pristiophoridae + Batomorphii) + *.
Characters: batoid-like shape; length to 2 m; preoral snout very short & truncate [C99]; mouth nearly terminal; $? nasoral groove present [dC96, 55(1)]; nostrils terminal bearing barbels on anterior margin; circumnarial grooves present [C99]; nostrils diagonal, with anterior incurrent & posteromedial excurrent [C99]; eyes dorsolateral [C99]; prominent subocular ridge [C99]; spiracles large; jaws highly protrusible; labial folds & cartilages prominent [C99]; teeth generally on broad base capped by an equally tall cusp; teeth without basal ridge [C99]; teeth usually recurved toward the inside of the jaws; 5 gill openings [C99]; spiracle large, but without valves [C99]; trunk dorsoventrally flattened [C99]; 2 dorsal fins, without spines [C99]; dorsal fins large; $? anal fin absent [dC96, 66(0)] [C99]; caudal vertebral axis bends ventrally & epaxial lobe doubled to create an effectively hypocercal tail [C99]; original hypaxial lobe is reduced [C99]; pectoral fins large, with triangular lobe overlying gill slits [C99]; scapulocoracoid with 2 condyles, one shared by pro- & mesopterygium [C99a]; marine only; feed on a variety of small bony fishes, crustaceans, cephalopods, gastropods and bivalves; capable of engulfing prey at high speed; ovoviviparous [C99].
Links: Genus Squatina; FAMILIES - Detail; Squatinidae; SQUATINIDAE; Squatinidae; Angelsharks. Squatinidae. Australian Angel Shark (Squatina australis); Familie Squatinidae (German); squatinidae Italian); Haie - Faszinierende Tiere - Engelshaie (German); Squatinidae images.htm; nomi.htm.
References: Compagno (1999) [C99]; deCarvalho 1996) [dC96]. ATW021013.
 Pristiophoridae: Saw sharks. Pliotrema, Pristiophorus.
Pristiophoridae: Saw sharks. Pliotrema, Pristiophorus.
Range: from the Late Cretaceous; presently 2 genera in coastal waters from South Africa to Japan & Australia
Phylogeny: Hypnosqualea:: Batomorphii + *.
Characters: Body shark-like, to 140 cm; snout elongated into long, flat blade with alternate large and small teeth weakly embedded on each side; one long barbel on each side [C99]; nostrils longitudinal, with anterior incurrent & posterior excurrent [C99]; nasoral groove absent [C99]; precerebral fossa present & roofed [dC96, 3(1)]; ectethmoid process present as cartilage [dC96, 8(2)]; prominent suborbital ridge [C99]; orbits dorsolateral [C99]; hyomandibular articular fossa composed of 2 horizontally arranged concavities [dC96, 14(1)]; mouth posterior to orbits [C99]; m. suborbitalis inserting on mandible via elongate tendon [dC96, 27(2)]; m. adductor mandibulae composed of lateralis components (not dorsal & ventral) [dC96, 25(1)]; $ m. constrictor hyoideus dorsalis inserts on both palatoquadrate & hyomandibula [dC96, 31(2)]; labial folds & cartilages reduced [C99]; teeth with basal ridges [C99]; gill openings 5 in Pristiophorus and 6 in Pliotrema; spiracles large, with valve [C99]; anterior basibranchials absent [dC96, 18(1)]; occipital hemicentrum absent [dC96, 17(1)]; dorsal fin spines absent [C99]; anal fin absent [C99]; supraneurals enlarged from abdominal region back [dC96, 46(2)]; $? (reversal) ventral caudal muscle bundle poorly developed [dC96, 53(0)]; caudal fin barely heterocercal [C99]; pectoral fins moderately large [C99]; 3 pectoral basals with separate condyles for articulation with scapulocoracoid [dC96, 38(2)]; $? (reversal) pectoral propterygium not extended anteriorly [dC96, 41(0)]; claspers with lateral spines [C99]; ovoviviparous [C99].
Links: FAMILIES - Detail; Haie - Faszinierende Tiere - Sägehaie (German); ordine_pristiophoriformi Italian); The orders of sharks (overview); WAsharkguide.pdf; Richard Ellis Gallery:; Reefquest.
References: Compagno (1999) [C99]; Compagno (1999a) [C99a]; deCarvalho 1996) [dC96]. ATW021013.
 Batomorphii: =Batoidea) rays, skates. Used as Torpedo + Raja.
Batomorphii: =Batoidea) rays, skates. Used as Torpedo + Raja.
Great flattening and elaboration of dorsal fin; precerebral fossa present & roofed [dC96, 3(1)]; ectethmoid process present as cartilage [dC96, 8(2)]; antorbital nasal capsules present [C99]; nasal capsules often laterally expanded [C99a]; $ antorbital cartilage joins propterygium and nasal capsule [Mc96, 7(1)]; orbits dorsal; upper eyelids fused to eyeball [C99]; $ upper eyelid lost [Mc96, 1(1)]; $ mm levator & depressor rostri present [Mc96, 8(1)]; palatoquadrate reduced; palatoquadrate suborbital articulation lost (i.e., $ palatoquadrate has no articulation with neurocranium [Mc96, 2(1)]), with increase of jaw mobility; pavement dentition common; sexual dimorphism (seasonal) with males having some biting teeth (apparently, the females find this amusing); m. suborbitalis inserting on mandible via elongate tendon [dC96, 27(2)]; m. adductor mandibulae composed of lateralis components (not dorsal & ventral) [dC96, 25(1)]; hyomandibular articular fossa composed of 2 horizontally arranged concavities [dC96, 14(1)]; $ ceratohyals reduced or absent [Mc96, 40(1)]; ceratohyals functionally replaced by pseudohyals derived from hyoid rays [C99] [Mc96, 3(1)]; enlarged dorsal spiracle; ventral gill slits; anterior basibranchials absent [dC96, 18(1)]; $ last ceratobranchial articulates with scapulocoracoid [Mc96, 4(1)]; occipital hemicentrum absent [dC96, 17(1)] [C99]; $ anterior vertebrae fused (synarcual) [C99] [Mc96, 5(1)]; dorsal fin spines absent; dorsal fins reduced or absent; anal fin absent; supraneurals enlarged from abdominal region back [dC96, 46(2)]; tail reduced or long and whip-like; 3 pectoral basals with separate condyles for articulation with scapulocoracoid [dC96, 38(2)]; propterygium anterior basal of pectoral fin) in contact with orbital process through antorbital cartilage; pectorals fused to side of head over external gill openings [C99]; pectoral propterygia larger than mesopterygia [C99]; $ pectoral girdle articulates or fuses with vertebral column through suprascapular [C99] [Mc96, 6(1)]; cartilaginous radials highly branched distally & extend to margins of pectoral fin (displacing ceratotrichia) [C99]; squamation sparse, with redevelopment of mucous cuticle and other glands, including toxins [K99]; some groups have electric discharges; omnivory common.
[1] Note that there is little or no overlap between the synapomorphies of [dC96] and [Mc96]. This is usually not a good sign. What is a very good sign is that the synapomorphies of [Mc96] are very close to traditional ideas of what makes a ray. The whole idea of cladistics is that it avoids the traps of intuitive classification schemes. However, cladistics has traps of its own. When the two schemes converge, we can be reasonably confident that we've got it right. [2] [S96] comes up with a significantly different topology, in which the Torpediniforms and all other rays form a clade to the exclusion of pristids and rhinobatoids. That being the case, I have not included his synapomorphies above. They are as follows: $ m obliqus inferior absent (reversal) [S96, 22(0)]; $ nasoral groove present [S96, 92(1)]. The latter character, as Shirai notes, is found in a number of unrelated lineages.
Links: elasmo.com
--Extant Batoids -- Guitarfishes, skates and rays; Encyclopedia Entry.
References: Compagno (1999) [C99]; Compagno (1999a) [C99a]; deCarvalho 1996) [dC96]; Kemp (1999) [K99]; McEachren et al. (1996) [Mc96]; Shirai (1996) [S96]. ATW021013.
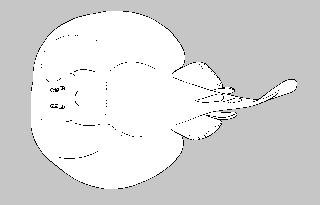 Torpediniformes: electric rays
Torpediniformes: electric rays
Small (10cm) to large (2m) electric rays. Batoids with pectoral fin greatly expanded and fused with head and trunk, forming oval or circular disk; mouth and gill slits ventral; eyes close-set and reduced or covered (4 species considered blind); nasal slits closely associated with eyes; kidney-shaped electric organs derived from branchial muscles near antero-distal margin of disk, often visible as hexagonal marking; tail short and stout; usually powerful electric shock used in predation; caudal fin well-developed and active in swimming; 0-2 dorsal fins with 1st closer base than tip of tail; cryptic coloration; ovoviviparous; inshore to benthic deepwater.
References: Moller 1995); Nelson (1984); Smith & Heemstra (1986).
Image: from Fishbase. ATW000618.
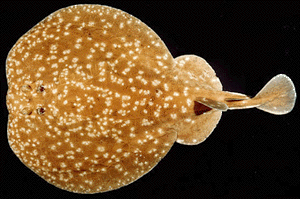 Torpedinidae: Hypnos, Torpedo. electric rays.
Torpedinidae: Hypnos, Torpedo. electric rays.
Range: From the Early Eocene. Presently found in Atlantic, Pacific & Indian Oceans.
Characters: rostrum absent or reduced; eyes small; jaws extremely slender; no labial cartilages; head with electric organs, developed from branchial muscles; disc truncated or emarginated anteriorly; 0-2 dorsal fins; caudal fin well developed; skin soft and loose; young hatch inside uterus; young born fully developed; swim by undulation of tail, not disk (as all rays & as opposed to skates).
Links: ray; FAMILIES - Detail (from Fishbase: as usual, Best on the Web); Torpedinidae; InterNevod: СЕМЕЙСТВО ГНЮСОВЫЕ (TORPEDINIDAE); ●ヤマトシビレエイ科 Torpedinidae/シビレエイ亜目 Torpedinoidei; Torpedinidae; GENSP, a New Species of Electric Ray ... abstract); Torpedoes, Skates, and Rays. Order Batoidei; The Shovelnose Guitarfish is a guitar-shaped ray with a broad ....
Image: Torpedo panthera © John Randall, reproduced by permission. ATW031121.
 Narcinidae: Diplobatis, Heteronarce, Narcine, Narke
Narcinidae: Diplobatis, Heteronarce, Narcine, Narke
Range: Atlantic, Indian and Pacific Oceans.
Characters: usually 30-50 cm; jaws stout; strong labial cartilages; rostrum present; eyes small; head equipped with powerful electric organs developed from branchial muscles; well developed caudal fin; dorsal fins
0-2; skin soft and loose; disc rounded anteriorly; marine primarily in shallow to off-shore waters of continental shelf regions, but some species known from upper continental slope.
Links: FAMILIES - Detail Fishbase: Best on the Web); Biological Batteries; Discopyge tschudii (Spanish); DEG - ELASMOSKOP Nr. 1/99 (German); Untitled; Quem são os Elasmobrânquios? (Portuguese).
Image: From Fishbase. ATW020220.
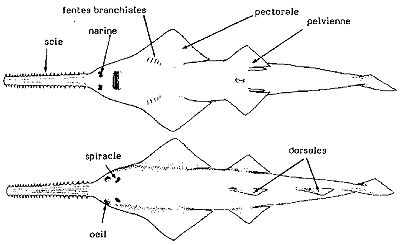 Pristidae: sawfish. Anoxypristis, Onchosaurus, Pristis, Propristis
Pristidae: sawfish. Anoxypristis, Onchosaurus, Pristis, Propristis
Characters: large (commonly ~5 m); head dorsoventrally flattened; saw-like rostra; "saw" from rostral projection of cranium; rostral projection strengthened by calcification; "saw" is 15-25% length of fish; two series of 16-32 large rostral tooth-like structures; cartilage of "teeth" encloses 3-5 longitudinal canals or ducts; rostral teeth are embedded in deep sockets (alveoli) of calcified cartilage; teeth consist of dermal denticles with continually growing bases; "teeth" slightly recurved, dorsoventrally compressed, with flattened posterior edge and slightly striate base; nares anterior to mouth and well separated; mouth ventral and rectangular; eyes and spiracle near the top of the dorsal surface; spiracles well-separated and posterior to orbits; teeth numerous and set in pavement dentition along jaws; gill slits on ventral surface; two dorsal fins, well-separated; tail not distinct from trunk; tail with longitudinal crest on each side; pectoral fins somewhat enlarged and fused to the head, but not reaching anteriorly to the level of the mouth; the whole body, including "sword" and fins, densely covered with small, ovoid, flat, relatively uniform denticles, without large tubercles; bottom-dwellers in tropical & subtropical shore <10m) areas; often fresh water or estuarine; rostra used to probe sand/mud or slash & stun fishes.
Links: Elasmo.com; Pristidae; Smalltooth Sawfish; and, of course, Fishbase; skates & rays (Russian).
Note: There are many rather good links on this group. ATW020427.
Sclerorhynchidae: Ischyrhiza, Odontopristis, Onchopristis, Ptychotrygon, Schizorhina.
Range: middle Cretaceous (Barremian) to Paleocene of Europe, Africa, North & South America.
Phylogeny: Batomorphii::: Rajiformes + *.
Characters: close swordfish analogs independently evolved from rhinobatoids [KA02]; rostrum tapers very gradually; superficial ophthalmic nerve run down dorsal canals in rostrum & buccopharyngeal nerve down ventral canals [KA02]; teeth replaced (coordinately in Schizorhina), rather than continuously growing as in living sawfish [KA02].
Note: the phylogenetic position of this group is rather arbitrary.
Links: Ptychotrygon; hunt2.html; Schizorhiza - a unique sawfish paradigm from the Difunta Group, ...; elasmo.com; Fossil Room; Cretaceous Fossils- Sharks and Rays Index an excellent tooth guide with references). One can find any number of good tooth sites by searching on the individual genera.
References: Kirkland & Aguillón-Martínez (2002) [KA02]. ATW030310.
Rajiformes: rays.
snout pointed; pectoral disc usually rhomboid; tail slender, set off from pectoral disc; caudal fin moderately well developed, reduced or absent; weak electrogenic organs at base of tail stalk (derived from caudal peduncle muscles); 0 to 2 dorsal fins; most with enlarged, thorn-like denticles ('bucklers') on skin, often with a row along midline of back; oviparous; almost exclusively marine; a few species live in shallow waters close to shore, but most live in deep water, on soft bottoms along continental margins, down 3000 m or more.
Links: egg-capsules of rays and skates. Animal Diversity Web: Rajiformes (not too useful on this taxon); ReefQuest Rick Martin's usual good job); Elasmo.com; Les Raies French). ATW000203.
 Starting from the front end, something significant happens to the rostrum. In the sawfishes, the rostrum is enlarged. It develops lateral "teeth" which may have sensory and/or attack functions. In this morph, the rostrum the rostrum encloses the precerebral fossa. By contrast, in rays, the rostrum is very short and broad. The precerebral fossa continues back, through the neurocranium, to a parietal fossa. If we accept that Hypnosqualea is a clade which has both ray and sawfish morphs at various points, we must accept that these two alternatives are somehow homologous. It is hard to see how this can be the case, but the overall unity of the Hypnosqualea is compelling. In both cases, separate ventrolateral and anterolateral cartilages on nasal capsules support the anterior of the head or attach to the propterygia. This may be the common denominator, as these unique cartilages may be oriented anteriorly, to support the rostrum of sawfishes, or laterally, to support the "wing" of rays. Labial cartilages are reduced or absent in all Hypnosqualea.
Starting from the front end, something significant happens to the rostrum. In the sawfishes, the rostrum is enlarged. It develops lateral "teeth" which may have sensory and/or attack functions. In this morph, the rostrum the rostrum encloses the precerebral fossa. By contrast, in rays, the rostrum is very short and broad. The precerebral fossa continues back, through the neurocranium, to a parietal fossa. If we accept that Hypnosqualea is a clade which has both ray and sawfish morphs at various points, we must accept that these two alternatives are somehow homologous. It is hard to see how this can be the case, but the overall unity of the Hypnosqualea is compelling. In both cases, separate ventrolateral and anterolateral cartilages on nasal capsules support the anterior of the head or attach to the propterygia. This may be the common denominator, as these unique cartilages may be oriented anteriorly, to support the rostrum of sawfishes, or laterally, to support the "wing" of rays. Labial cartilages are reduced or absent in all Hypnosqualea.






