|
|
Tracheophyta |
| Plants |
Tracheophyta - 2 |
Tracheophyta - 2
Anatomy
 We have reorganized this section several times, generally ocillating between phylogenetic anatomy and anatomical phylogeny. We ultimately reached the conclusion that there is no reasonable way to organize this material. We suspect that Kenrick & Crane (1997) found the same problem a dozen years ago, since they ultimately describe the anatomy of early plants several times, using various different schemes of organization. In fact, we strongly recommend Kenrick & Crane (1997) to the interested reader. The review below is written -- as is most of Palaeos -- solely for the purpose of educating ourselves. For that purpose, we will take an anatomical approach, trying to make sense of the evolution of the various organ types using the consensus phylogeny. Kenrick & Crane (1997), who invented the now consensus phylogeny, do this far better than we could ever hope to do.
We have reorganized this section several times, generally ocillating between phylogenetic anatomy and anatomical phylogeny. We ultimately reached the conclusion that there is no reasonable way to organize this material. We suspect that Kenrick & Crane (1997) found the same problem a dozen years ago, since they ultimately describe the anatomy of early plants several times, using various different schemes of organization. In fact, we strongly recommend Kenrick & Crane (1997) to the interested reader. The review below is written -- as is most of Palaeos -- solely for the purpose of educating ourselves. For that purpose, we will take an anatomical approach, trying to make sense of the evolution of the various organ types using the consensus phylogeny. Kenrick & Crane (1997), who invented the now consensus phylogeny, do this far better than we could ever hope to do.
It is worth noting that the traditional organization used by paleobotanists is neither anatomical nor phylogenetic, but temporal. As we've noted elsewhere, there is some tendency for plants of all lineages to develop the same sorts of structures at about the same time. So for example, the density of stomata increase through the Devonian in all groups. The development of monopodial branching, the tree body plan, reduction of the gametophyte, the evolution of leaf structures, and the differentiation of roots all occur in rough synchrony across land plant groups. It is possible, but rather unlikely, that our ideas of tracheophyte phylogeny are grossly mistaken. It is more likely that broadly parallel (homoplastic) evolution actually occurred and that it occurred because all land plants were experiencing parallel changes in their environment. For this reason numerous articles have tried to make sense of tracheophyte evolution in terms of secular change in broad environmental factors. We like this approach, but apply it with caution. It is too easy to slip from this type of analysis into purely scenario-based speculation. We really don't know all that much about terrestrial environments in the Middle Paleozoic (Silurian and Devonian), and the fossil record of plants at the time is only good for the Early and Late Devonian.
Another common approach is to view the plant body plan as simply having fewer possibilities than (for example) the animal body. Viewed from this perspective, early plant evolution is the constrained development of a delicate balancing act between "[t]he functions essential for nearly all independently growing terrestrial plants — desiccation resistance, structural support, hydraulic and solute transport, and photosynthesis." Boyce (2008).
However, this pattern of evolution is not necessarily due to some inherent limitation of plant genetics. The dynamic of early evolution in terrestrial plants differs from that in terrestrial animals in several fundamental ways. Early terrestrial animals were all motile predators or detritovores. Terrestrial herbivores were either non-existent or of minimal significance right into the Permian. Labandeira & Allen (2007). So animals chased each other around for food or sex, from the very start. What is more, they chased each other around in an environment already partitioned by plants. Shear & Selden (2001). In contrast, early terrestrial plants were sessile. They had no macroscopic predators at all and were expanding into an environment which possibly abiotic or, at the most, was only very thinly conditioned by other living organisms. Therefore it is perhaps not surprising that -- in general -- terrestrial animals evolved more by environmental specialization through genotypic plasticity; while early plants tended towards large monotypic stands which created their own environment and by greater reliance on phenotypic plasticity. The kinds of constraints imposed by the two modes of terrestrial evolution were quite different [1].
But this is all rather high level speculation. Let's look at the practical details.
The Roots of Roots
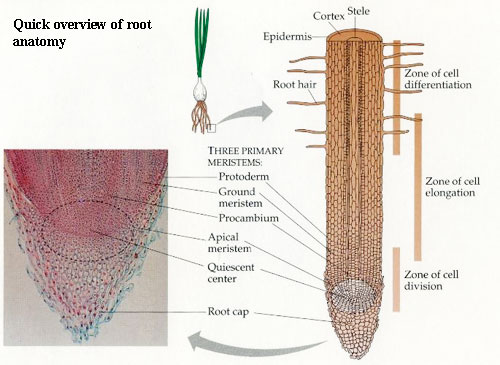 To decide how roots evolved, we must decide what roots are. This has proven difficult. The most careful approach to a modern definition is that of Raven & Edwards (2001). Rather than provide a definition, Raven & Edwards assert that a root is any axial structure of the sporophyte which has all or most of the following characteristics: (1) direction of growth (on average) within 90° of gravitational field; (2) growth away from light (negative phototropism); (3) elongation growth is strictly apical, without bifurcation of the meristem (root cap); (4) possession of a histologically distinct root cap; (5) endodermis; (6) protostele (sometimes with a pith); (7) endogenous origin of lateral roots, i.e., branching by the initiation of new meristem growth region from the stele. Of these characters, (2), (4), (5), and (6) aren't very useful for fossils or have too many exceptions. Endodermis (5) and protostele (6) may also be plesiomorphic for tracheophytes. Characters (3) and (7) are two related aspects of meristem development. Root meristem does not divide, nor does it branch superficially. A root has true monopodial (single main axis) development and branching occurs by new growth continuous with the central vascular strand.
To decide how roots evolved, we must decide what roots are. This has proven difficult. The most careful approach to a modern definition is that of Raven & Edwards (2001). Rather than provide a definition, Raven & Edwards assert that a root is any axial structure of the sporophyte which has all or most of the following characteristics: (1) direction of growth (on average) within 90° of gravitational field; (2) growth away from light (negative phototropism); (3) elongation growth is strictly apical, without bifurcation of the meristem (root cap); (4) possession of a histologically distinct root cap; (5) endodermis; (6) protostele (sometimes with a pith); (7) endogenous origin of lateral roots, i.e., branching by the initiation of new meristem growth region from the stele. Of these characters, (2), (4), (5), and (6) aren't very useful for fossils or have too many exceptions. Endodermis (5) and protostele (6) may also be plesiomorphic for tracheophytes. Characters (3) and (7) are two related aspects of meristem development. Root meristem does not divide, nor does it branch superficially. A root has true monopodial (single main axis) development and branching occurs by new growth continuous with the central vascular strand.
Thus, the short explanation is that a root is something that grows like a tree, only downward. That being the case, you will be unsurprised to learn that real roots and real trees developed about the same time -- probably no earlier than the Middle Devonian. Kenrick & Crane (1997a); Bateman et al. (1998); Raven & Edwards (2001). The main technical obstacle to root and tree evolution seems to have been finding a reliable method for putting parts of the active, apical meristem to sleep for a while, then waking them up to form secondary axes continuous with the original stele. We'll talk a little more about this problem when we get to branches.
Root-like impressions are known from the Late Silurian. Structures that really look like roots are first found associated with Early Devonian tracheophytes. However, even these fossils are probably the remains of rhizomes. Rhizomes are not roots. They are part of the main trunk of the plant which grows more or less horizontally along the surface of the earth. Typically, rhizomes generate nodes at intervals. From these nodes grow aereal branches and/or root hairs (or even true roots in more recent plants). Mosses have essentially the same system.
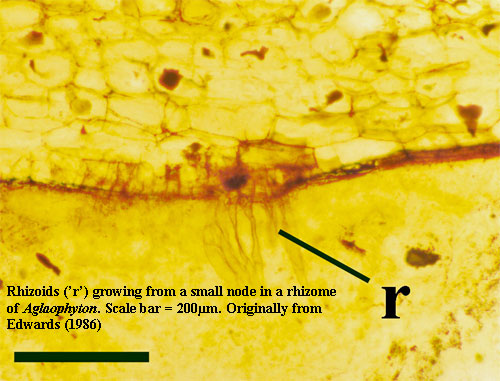 Essentially all land plants have root hairs (rhizoids) for absorbing water and soil ions. Rhizoids are one or a few cells wide and are thus rarely preserved in fossils. However, they are common where conditions permit preservation in detail, as in the Rhynie Chert. Kenrick & Crane (1997). "Many germinating spores in the Rhynie chert are associated with microbial mats that contain cyanobacteria and other microbes. In modern microbial mats extracellular polymeric substances are important components of the mat that are involved in attaching microbes to the substrate and forming a matrix around the organisms" Taylor et al. (2005).
Essentially all land plants have root hairs (rhizoids) for absorbing water and soil ions. Rhizoids are one or a few cells wide and are thus rarely preserved in fossils. However, they are common where conditions permit preservation in detail, as in the Rhynie Chert. Kenrick & Crane (1997). "Many germinating spores in the Rhynie chert are associated with microbial mats that contain cyanobacteria and other microbes. In modern microbial mats extracellular polymeric substances are important components of the mat that are involved in attaching microbes to the substrate and forming a matrix around the organisms" Taylor et al. (2005).
Fungal associations important in early plant history Kenrick & Crane (1997a). Probable functional predecessor of roots. Raven & Edwards (2001). "Fungal associations are widespread in gametophytes of pteridophytes, liverworts and hornworts, but are lacking in mosses (Duckett et al. 1991). Fungal hyphae facilitate absorption and translocation of minerals, water and organic molecules within the plant. In nature, heterotrophic subterranean gametophytes of pteridophytes rely exclusively on endophytic fungi for nutrient uptake." Renzaglia et al. (2000).
BUT: "Rhynie Chert tracheophytes and protracheophytes -- all of roughly similar axis diameter to extant Psilotum ... possess rhizoids (Aglaophyton, Horneophyton, Rhynia), other rooting structures (Asteroxylon), or rhizomatous growth (Aglaophyton, Asteroxylon, Nothia, Rhynia) ... ." Boyce (2008).
Aglaophyton (and some other Rhynie plants) have underground axes bearing rhizoids; these axes have arbuscular mycorrhizas, but mycorrhizas also occur on above-ground axes. Raven & Edwards (2001)
Unicellular rhizoids present in all Rhynie plants, but are rarely preserved in other fossils. Kenrick & Crane (1997). Image at 66. On observe dans les axes de certaines espèces (comme Aglaophyton) des champignons symbiotiques rappelant les mycorrhizes des racines des arbres actuels. DuBuisson et al. (2005).
In these very primitive land plants in which there was little differentiation between the different parts. They had simple shoots rise from a creeping axis which hardly differs in structure from the upright shoots themselves. Instead of roots they had horizontal stems, connected with the soil by root hairs.
Diameter seems to help in growth rate, cytoplasmic streaming, life-span and structural maintenance; hurts in surface/volume. Raven & Edwards (2001).
Rhyniophytes: still no roots. Raven & Edwards (2001).
Rhizoids of Rhynia are borne on hemispherical projections near the base of presumed aerial axes. Raven & Edwards (2001).
Nothia included by some, but its a lycopsid per Crane et al. (2004).
Eutracheophyta: Just as nearly all vascular plants have stomata, nearly all have an endodermis with a waterproofing casparian strip of lignin and suberin (Schreiber 1996; Raven and Edwards 2001), whether or not the absorbing organs are true roots or are limited to stems. An interesting exception is Lycopodium, where an endodermis is lacking in roots but present in stems; however, an “endodermoid layer” in the roots seems to have the equivalent function (Raven 1984). The earliest appearance of an endodermis in the fossil record is from the Carboniferous (Raven 1984), but it likely originated at least as early as the split of the tracheophytes into the lycophyte and euphyllophyte clades (fig. 1). Sperry (2003). BUT endodermis (seen only in roots of higher plants, but also in stems of Psilotum), Lecture.
Lycophyta: "Roots in zosterophylls, "stigmarian" roots arborescent lycopsids (Lepidodendron) and Isoetes may all be morphologically analogous leaves. "This root-like organ develops into the 'stigmarian root system' bearing 'stigmarian rootlets'. and the stigmarian axis and its rootlets is considered homologous to the shoot system (Rothwell, 1984; Rothwell and Erwin, 1985). This view is supported by the presence of a pith cavity in the stigmarian root system, and by the exogenous production of lateral organs (stigmarian rootlets) (Stewart and Rothwell, 1993; Taylor and Taylor, 1993). The 'stigmarian rootlets' have thus been interpreted as morphologically equivalent to leaves, and are closely similar in anatomy to the roots of the extant Isoetes spp. where, as one might expect of modified leaves. the roots have a major role in CO2 uptake by the plant." Raven & Edwards (2001). R&E argue that absence in Silurian and Early Devonian lycophytes means independent origin, but note that this "requires that such features of roots as a root cap, and endogenous origin of laterals evolved independently from the original relatively undifferentiated axis of the lycophytes and the euphyllophyte."
The anaromy of rootjng systems is unknown in nearl)' all of the early fossil lycopsids and zo .. uel'oph)·IL~.K&C
Zosterophylls: The published information on roots or root-like structures in the early Devonian (Pragian-Emsian) (Gensel et al., 2001) has been reviewed, and new data presented on Lower Devonian fossils from Bathurst Island, Arctic Canada, and from New Brunswick and Gaspe, Canada. These plants with root-like structures are zosterophyllophytes or lycophytes sensu lato." These had "(1) The rooting structures are attached to aerial ones, but have no emergences or microphylls. (2) The rooting structures are generally much narrower in diameter, and shorter, than are the aerial axes that bear them. (3) The rooting structures are either unbranched or are irregularly branched; this contrasts with the more predictable branching of the aerial axes. (4) The rooting structures may have a sinuous or delicate appearance. (5) The rooting structures proceed in a direction opposite to that in which the aerial axes grow. These authors point out that characteristics 2-5 are also applicable to plants with naked axes, and that the rooting structures show no evidence of root caps or indisputable root hairs; furthermore, the anatomy of the rooting structures is not yet known. Raven & Edwards (2001). Authors argue unconvincingly that these and Drepanophycus qujingensis don't have roots since no evidence of root cap, endodermis, etc. none of which is to be expected in fossils lacking the spectacular preservation of Rhynie Chert specimens.
"[Bathurst Is.] Zosterophyllum sp. nov. with tufted rooting structures which occur as descending naked axes from plants with dense aggregations of branching aerial shoot systems." Raven & Edwards (2001).
"The Quebec and New Brunswick fossils comprise Crenaticaulis verruculosus and Sawdonia ornata: C. verruculosus has smooth, spinous. slender (0.5 mm wide) rooting structures departing at right angles to aerial or rhizomatous axes in regions where there are otherwise no branches (Gensel et al., 2001). Sawdonia ornata fossils have [similar] structures " Raven & Edwards (2001).
Rhizomatous axial systems; ? Aerial axes that are dichotomous or pseudomonopodial, an elliptical exarch protostele; ? Axes with randomly arranged spines in some genera; ? Cauline (occurs on the axis) globose or reniform sporangia, trilete spores G.
Lycopsids: Lycopodiophyta: "The least inclusive clade containing Lycopodium clavatum L. 1753, Huperzia selago (L.) Schrank & Mart. 1829, Isoëtes lacustris L. 1753, and Selaginella apoda (L.) Spring 1840." That's the crown clade. For this clade: "association of a single axillary or adaxial sporangium with a sporophyll; absence of vasculature in the sporangium; metaxylem tracheids pitted; root stele bilaterally symmetrical, with phloem located on only one side of the stele (but there are a lot of missing data for fossils outside the crown, so this trait may be synapomorphic of a more inclusive clade); crescent-shaped root xylem (but there are a lot of missing data for fossils outside the crown). The following characters are synapomorphies of this crown clade relative to other crowns but are apomorphic at a more inclusive level when fossils are considered (Kenrick & Crane, 1997: Fig. 6.18, Table 7.2): microphylls (“lycophylls”; Schneider & al., 2002; Pryer & al., 2004a); exarch xylem differentiation in stem (Kenrick & Crane, 1997; Doyle, 1998; Schneider & al., 2002); stellate xylem strand in stem; reniform sporangia with transverse dehiscence (Doyle, 1998). This list is not exhaustive; see Kenrick & Crane (1997: Table 7.2) and DiMichele & Bateman (1996) for other synapomorphies listed under Lycophytina and Lycopsida." Cantino et al. (2007).
"earliest roots in the fossil record have diameters ranging from 3 mm at their attachment to less than 0.7 mm in the smallest branches recovered." Raven & Edwards (2001). Early Devonian lycopsids.
Asteroxylon mackiei is one of the earliest known Lycopods from the Rhynie Chert with microphyllous leaves; rhizome creeping, naked, developed repeatedly bi-furcating root-like structures (these were not true roots as the root-cap was absent),
Baragwanathia: but we have little knowledge of the underground structures which, it is widely believed, such plants must have possessed if they were to have been adequately anchored, and to have had a sufficient capacity for uptake of water and soil-derived nutrients ... It is clear from the size of the erect aerial axes of Baragwanafhia that substantial below-ground structures would be needed." Raven & Edwards (2001).
"The Bathurst Island fossils (Gensel et al., 2000) comprise Drepanophycus and Bathurstia spp. with aerial axis-borne rooting structures scattered along what appear to be trailing or rhizomatous structures, or forming parts of the so-called 'K-branching' structures" Raven & Edwards (2001).
Rayner argues that Drepanophycus spinaeformis had roots on the basis of the branching pattern and the attitude in the sediment of the smooth axes in relation to the bedding plane (Schweitzer, 1980). However, there is no anatomical evidence (e.g. endogenous branching) to support this view. Li and Edwards have looked at Drepanophycus qujingensis (Li and Edwards, 1995), was shown to have roots, i.e. structures borne by both fertile and sterile leafy shoots, which branch dichotomously at least five times. The roots appear to be inserted randomly, and the largest branching root system extends up to 30 mm from the stem. The diameter of the roots is about 3 mm at their attachment and rapidly decrease with branching; the smallest branches recovered have a diameter of less than 0.7 mm (Fig. 37D of Li and Edwards, 1995), and it is likely that the unpreserved, more apical, portions had an even smaller diameter. It is of interest that these roots were borne on leafy axes which were presumably above-ground but were probably prostrate, as the rooting system itself extends down into the rock, at an angle to the bedding plane in which the leafy axes were exposed. Raven & Edwards (2001).
Isoetes only survivor with "rootlets" of the type developed in tree-like lycopsids. Donoghue (2005).
"anatomical studies of arborescent lycopsids raise certain matters with regard to their physiology (Phillips & DiMichele, 1992). The lack of a clear phloem connection between root and shoot, the generally limited phloem throughout the plant’s aerial shoot, the leaf-like rootlets borne on the stigmarian axes, and the long leaves on stems and cones, are all consistent with extremely localized use of photosynthate and perhaps even self-supporting root systems in terms of carbon fixation." DiMichele & Gastaldo (2008).
Stem Euphyllophyta: Euphyllophyta crown group: Synapomorphies (relative to other crown clades). — Roots with monopodial branching and endogenous lateral roots (Schneider & al., 2002); ... (these features characterize the earliest members of /Pan-Euphyllophyta and were modified in most extant representatives); Cantino et al. (2007).
Psilophyton "emerged from rhizomatous mat". K&C
Furthermore. the zosterophyllophyte-lycophyte sensu lata clade possessed root-like structures in the Lower Devonian, although there is no corresponding evidence for root-like structures on euphyllophytes at this time. The earliest convincing euphyllophyte roots occur in the Middle Devonian cladoxylalean Lorophyton goense. thought to derive from trimerophytes (Fairon-Demart and Li, 1993) where bifurcating structures arise in tufts from a swollen stem base. This is consistent with the polyphyletic origin of roots, a point considered in more detail below. Raven & Edwards (2001).
[Theory] suggest a height for Archaeopteris of 10-30 m. Corresponding to this increase in height is an increased plant biomass per unit land area and depth of penetration of roots sensu lato. ... there is independent evidence of an increasing depth of penetration of roots ... up to 2 mm in diameter and up to 0.9 m long in the Emsian (late Early Devonian) have been found." Raven & Edwards (2001).
The "Original Condition"
Bryophytes: "Possibly the earliest upright cylindrical structures among embryophytes were erect gametophores embedded in thalloid structures. Because the cylindrical structures lacked specialized vascular tissues or hypodermal steromes, the diameter and height of such columns would have been severely restricted by potential conductance (105) and relied on turgor pressure to maintain an upright stance. The height ... may have conferred greater dispersal potential than that possessed by forms sporulating directly from the thallus surface, allowing spores to reach uncolonized areas beyond the dense, extensive clonal mat." Huh? Bateman et al. (1998).
Mosses transitional in having erect gametophyte tissues with conducting tissue. Bateman et al. (1998).
Polyphenols in hydroid walls. Sperry (2003).
No branching in sporophyte, then some dichotomous. Kenrick & Crane (1997: 299)
No synapomorphies for Pantracheophyta. "An independent sporophyte and multiple sporangia are listed by Kenrick & Crane (1997: Table 7.2) as synapomorphies of Polysporangiomorpha (a slightly less inclusive clade than /Pan- Tracheophyta ; see Synonymy) but only the latter would be a synapomorphy of Polysporangiomorpha if it were given an apomorphy-based definition based on the etymology of the name. The order in which the two features evolved is not known. Sunken archegonia are also cited as a possible synapomorphy of Polysporangiomorpha by Kenrick & Crane (1997: Table 7.2), but it is unknown whether sunken archegonia evolved before or after multiple sporangia. Moreover, sunken archegonia also occur in Anthocerophyta (op. cit., Fig. 3.33, pp. 63–64) and thus may be a synapomorphy of a more inclusive clade if hornworts are the closest extant relatives of tracheophytes (e.g., Qiu & al., 2006b). Synonymy. — The name Polysporangiomorpha (poly- sporangiophytes) sensu Kenrick & Crane (1997: Table 7.2)" more or less. Cantino et al. (2007).
Early loss of dessication-resistance and development of homoiohydry (??). Sperry (2003).
Stems: In these very primitive land plants in which there was little differentiation between the different parts. They had simple shoots rise from a creeping axis which hardly differs in structure from the upright shoots themselves. Instead of roots they had horizontal stems, connected with the soil by root hairs.
La dispersion des spores peut être favorisée par une grande taille verticale. DuBuisson et al. (2005).
Vascular Tissue: There are also a number of related forms, like Aglaophyton, apparently not even true tracheophytes, other basal tracheophytes had G-type. Kenrick & Crane (1997a). Not much contribution to mechanical strength. Bateman et al. (1998).
"Aglaophyton and Horneophyton, [lack] tracheids with well-defined thickenings." Kenrick & Crane (1997a); Bateman et al. (1998). Such tracheid-like structures as they had were centrally located and seem to have evolved to with stand negative pressure of transpiration, rather than support the plant. Bateman et al. (1998). Central vascular strand almost indistinguishable from bryophytes. Kenrick & Crane (1997).
"Tissue differentiation within the seta of many mosses is quite pronounced and shows similarities to early fossil vascular and nonvascular plants, including the possession of a central conducting strand and a peripheral cortical layer of thickwalled cells. There is a central region of elongated and unthickened cells, possibly conducting tissue, surrounded by a poorly preserved, presumably parenchymatous, tissue and a layer of thickwalled epidermal cells (Halle 1916a, 1916b, 1936a). No thick-walled cortical tissue has been observed." Also applies to branches. Kenrick & Crane (1997). Evidence from the fossil record indicates that cells with helical or annular thickenings are more general than pitted cells because many early vascular plant fossils possess tracheids with only helical thickenings (Kenrick and Crane 1991). Well-defined wall thickenings are absent in the early polysporangiophyte fossil Horneophyton." Kenrick & Crane (1997).
They had simple shoots rise from a creeping axis which hardly differs in structure from the upright shoots themselves. These shoots or stems often branched in a simple manner, forking into two, and then into two again. Donoghue (2005). The whole plant was generally less than 50 cm in height. Basal tracheophytes developed the branched sporophyte. That is, "enlargement and branching of the sporophyte preceded the acquisition of tracheids." Dichotomous branching characterizes the polysporangiophytes. More spores per fertilization event and increased size. Donoghue (2005).
"Elements" of auxin metabolism known from the early embryophyte grade. Kenrick & Crane (1997a).
Protracheophytes still maintained largely by turgor pressure. Cortex took the strain. 20-30 cm max height. Bateman et al. (1998). However, "plant axes in the size range of smaller cooksonioid axes can rely exclusively on turgor from the hydrostatic core (Niklas 1997). Nonetheless, a stereome is preserved in some of the narrowest cooksonioids." Boyce (2008).
"The simplest conduits were thin and smooth-walled microperforate tubes of the now extinct protracheophytes (fig. 3A; Aglaophyton, Horneophyton; Kenrick and Crane 1991). These resemble the hydroids of some mosses and may have a common ancestry (fig. 1; Mishler and Churchill 1984; Kenrick and Crane 1997a; but Ligrone et al. 2000). These fossil tubes do not appear to have been lignified but may have been impregnated with lignin-like polyphenols, as are extant hydroids." Sperry (2003). Gametophyte of Aglaophyton also had conducting tissue. Taylor et al. (2005).
"pitting is absent from the tracheid cell walls of many basal taxa (Kenrick and Crane 1991). In these early fossils, all tracheids have conspicuous helical or annular thickenings that strongly resemble the early-formed protoxyem elements of extant groups ...
"The persistence of this plesiomorphic cell type in the protoxylem of extant vascular plants is probably strongly linked to functional constraints. In xylem tissues, cell elongation cannot occur by further growth after differentiation because metabolic death must occur before the cell is able to function. In vascular plants, most differentiation of primary tissues, including metaxylem, occurs after a period of cell elongation at a distance below the apex. This constraint means that the developing metaxylem tissue is unable to immediately supply the metabolically demanding growing point with xylem product (e.g., water and mineral nutrients). The role of the protoxylem is to ensure an uninterrupted supply of xylem product to the apex, and the protoxylem achieves this by early differentiation within the zone of tissue elongation immediately below the apex. Ultimately, many of the protoxylem cells become nonfunctional through severe distortion by cell elongation in the neighboring tissues, and the role of supplying water is taken over by the differentiating metaxylem with its more robust, heavily lignified, and pitted secondary walls. During the brief functional period of protoxylem, the helical-annular thickenings serve a dual role in resisting the lateral collapse (cavitation) of the cell walls while allowing axial elongation of the cell through stretching in response to growth of the surrounding tissues (Bailey 1953). The critical role of protoxylem in vascular plant ontogeny explains the persistence of the plesiomorphic helical-annular thickened cell wall and its widespread occurrence among extant groups." Kenrick & Crane (1997: 300)
Branches: Branching "set the stage" for development of more advanced vascular tissues. Donoghue (2005). [branching connected the vascular system to sites of transpiration and photosynthesis, thus creating need for tracheids] Branched sporophyte and multiple sporangia developed at the avascular level (Aglaophyton, Horneophyton). Kenrick & Crane (1997).
Branching pattern irregular [lack of long-range order]. Kenrick & Crane (1997). "Among early polysporangiophytes, branching is relatively simple (Rothwell 1995, see above) and may contain a significant stochastic component (Niklas 1982). A series of more complex and increasingly deterministic branching systems appear early within the major clades of vascular plants." Kenrick & Crane (1997: 298)
Leaves: External surface not elaborated for additional photosynthetic area as in some early embryophytes, Boyce (2008).
These ancient plants lacked leaves, Bateman et al. (1998). Aglaophyton and Horneophyton lacked leaves Kenrick & Crane (1997a).
Stomata: Stomata probably already present. Known from both Aglaophyton and Horneophyton. Kenrick & Crane (1997).
Stomatal density increase in all lines? through Devonian. Raven & Edwards (2001).
Many hornworts and mosses have stomata, but they are absent in liverworts. Benton & Harper (1997).
Sporangia: "in basal polysporangiophytes, they are often solitary at the ends of the main branching system, for example in Aglaophyton (Figure 4.7), Cooksonia" Kenrick & Crane (1997).
... sometimes the shoots terminated in spore capsules called sporangia. ... lacked seeds, and flowers.
Aglaophyton and Horneophyton branched, nutritionally independent sporophyte. Bateman et al. (1998). "two fundamental growth forms predominate in basal tracheophytes: (i) green epiterrestrial forms with irregular upright lamellae and (ii) thick, fleshy subterranean forms with a fungal symbiont. The latter type, exemplifed by Huperzia (figure 1a), Lycopodium, Diphasiastrum, Phegmariurus, Psilophytes (figure 1b), Botrychium and Ophioglossum, is generally considered ancestral. These gametophytes may persist for several years and they have the capacity to produce multiple sporophytes and form abundant regenerants." Renzaglia et al. (2000).
Aglaophyton sporangia are terminal and produce trilete spores.Taylor et al. (2005).
Unlike those of pteridophytes, gametangia of mosses and liverworts are stalked and extend from the epidermal surface (Bold et al. 1987). Gametangia of basal pteridophytes are never stalked and are either entirely sunken within the thallus or form conspicuous epidermal mounds (Bierhorst 1971). Renzaglia et al. (2000).
"In vascular plants the sporophyte is initially dependent on the gametophyte (its foot absorbs nutrients from the gametophyte) but develops complete physiological independence during early ontogeny (Bierhorst 1971). In liverworts, horn worts, and mosses, the sporophyte remains in intimate contact with gametophytic tissues during spore production." Kenrick & Crane (1997).
"The basal members of the Polysporangiomorpha clade are “protracheophyte”-grade genera such as Horneophyton and Aglaophyton. They exhibit branched, independent sporophytes isomorphic with gametophytes that possess sunken archegonia." Bateman et al. (1998); and multiple sporangia above this level. Kenrick & Crane (1997). That is, "transition to sporophyte dominance moved through a stage in which gametophyte and sporophyte phases were more or less similar in structure (so-called isomorphic alternation of generations." Donoghue (2005) (citing K&C). Contra Boyce (2008). [and Taylor et al. (2005)?]
Horneophyton unique in having branched sporangium. Kenrick & Crane (1997).
Aglaophyton and Horneophyton had terminal sporangia. Bateman et al. (1998). Horneophyton even retained a columella, a pillar of sterile tissue in the sporangium which supports the spore capsule. This is a feature typical of mosses, but virtually unknown in tracheophytes. Bateman et al. (1998).
the Rhynie chert plants can also reproduce by various asexual meansTaylor et al. (2005). All of the Rhynie chert plants can reproduce both sexually and by vegetative methods, and thus clonality may have served as the primary method of species invasion into new niches (54). Sections of rhizomes that supported a single sporophyte suggest that fragmentation and dieback may have been an important form of vegetative reproduction, as in some modern clonal plants (55). Other structures in the fossils that may have functioned in asexual reproduction include reduced branches as fragmentation propagules, bulbils (Fig. 2Q), and bulges containing poorly organized vascular tissue (56).Taylor et al. (2005).
"Terminal sporangium attachment on longcr axes is the general condition in polysporangiophytes." Kenrick & Crane (1997).
Gametophyte: "Mature gametophytes [of Aglaophyton] consist of a fleshy protocorm attached to the substrate by basal rhizoids; arising from the upper surface are one to several upright gametangiophores bearing multiple gametangia. Stomata are present on the upper surface of the protocorm and gametangiophore, and endomycorrhizal fungi extend throughout the gametophyte. Gametophytes are unisexual, producing either antheridiophores or archegoniophores. There is no evidence that gametophytes later become hermaphroditic. The sexual dimorphism of the Rhynie chert gametophytes is inconsistent with theoretical ideas about the haploid phase of early land plants." Taylor et al. (2005).
Earliest vascular plants had elaborate gametophyte. "Early gametophytes ... are more complex than in living plants and have branched stems bearing sexual organs on terminal cupor shield-shaped structures ... . Archegonia (female gametangia) are flask-shaped with a neck canal and egg chamber, and are sunken as in hornworts and most vascular plants (Fig. 2c). Antheridia (male gametangia) are roughly spherical, sessile or with a poorly-defined stalk, and superficial (Fig. 2b). Gametophytes are very similar to associated sporophytes, and shared anatomical features (waterconducting tissues, epidermal patterns, and stomates) have been used to link corresponding elements of the life cycle. ... after the development of a simple, unbranched, ‘parasitic’ sporophyte among early land colonizers ... there was elaboration of both gametophyte and sporophyte in vascular plants. ... The small, simple, often subterranean and saprophytic gametophytes of [lycopsids] and ferns ... result from morphological loss. ... gametophyte reduction was independent in clubmosses and the fern–seed plant lineage." Kenrick & Crane (1997a). [use image?]
Horneophyton with sunken archegonia (probably -- ID of gametophyte is not 100% certain). Kenrick & Crane (1997)
"Antheridial development is essentially identical to archegonial development in pteridophytes (figure 4c) (Bierhorst 1971). The process begins with a transverse division in an epidermal cell. The outer cell gives rise to a sterile jacket and the inner cell forms the spermatogenous tissue. So similar are these division patterns in the two sex organs of pteridophytes that it is virtually impossible to differentiate antheridia from archegonia in early stages of organogenesis. Moreover, in protandrous lycophytes, when the transition between male and female sex organs occurs, it is possible to find bisexual gametangia that contain developing sperm in the `neck region’ and an egg cell at the base (figure 5) (K. S. Renzaglia and D. P. Whittier, unpublished data)." Renzaglia et al. (2000). Compare "The shape and general appearance are like that of the exosporic gametophytes of living nonseed vascular plants. The first cell division of the gametophyte is transverse and divides the globular mass into nearly equal segments (Fig. 2 B and C). The outer cell continues to divide to form a mass of cells that will ultimately give rise to the gametophyte proper (Fig. 2 D and E); the inner cell also divides a few times to form a small cell mass that remains within the confines of the spore wall. Oblique cell divisions of the outer cell give rise to an apical cell that subsequently divides to form cells of two types (Fig. 2F). On the outside is an outer ring of slightly flattened cells that will form the epidermis; on the inside is an inner core of cells that are more isodiametric in outline. During elongation, apical cells of the inner core continue to divide so that the gametophyte becomes teardrop-shaped and �10 cell layers thick" Taylor et al. (2005). Taylor et al. emphasize that gametophores are morphologically very distinct and never hermaphroditic. This appears specialized compared to the pattern in lycophytes described by Renzaglia et al.
"Gametophytes of Lyonophyton are unisexual ... nor is there any evidence that gametangia are mixed on a single gametangiophore. Gametangiophores are morphologically distinct ... . Mature antheridiophores are upright, unbranched, and �~2.0 cm long. The distal ends are cup-shaped and lobed (Figs. 1 and 2K) and produce 10–40 antheridia, each spherical and ≤600µ in diameter. Antheridia are stalked and columellate (Fig. 2L), with delicate parenchyma strands extending from the columella to the inner wall of the antheridium; on the distal surface, a small pore is formed in mature antheridia through which flagellated gametes (Fig. 2M) are released. The distal ends of the archegoniophores consist of several closely spaced dichotomies (Fig. 1), each possessing conducting strands. The tip is slightly expanded with a shallow central depression. Archegonia are slightly sunken on the inner surface of the depression or are subterminal along the axis just beneath the tip. Each archegonium is hemispherical and consists of �8–10 cell rows, with each row one to three cells thick and up to six cells high (Fig. 2N)." Taylor et al. (2005).
"A cutinized epidermis covers both the antheridophores and archegoniophores; beneath this layer is a two-celled hypodermis. To the inside of this layer is a one- to two-cell-thick zone of slightly opaque isodiametric cells (Fig. 2H), some of which contain arbuscules (Fig. 2I) of the endomycorrhizal fungus Glomites (17). Hyphae enter the gametophyte at the base in the region of the rhizoids and extend through the intercellular spaces of the cortex (Fig. 2H)." Taylor et al. (2005).
"Free-living gametophytes are known for three of the Rhynie chert macroplants (Aglaophyton, Rhynia, and Horneophyton); to date, only antheridiophores are known for Nothia. All gametophytes consist of unisexual, axial, and upright structures (gametangiophores) that develop from the distal surface of a protocorm; on the base are tufts of rhizoids. Gametangiophores are small (�5 cm tall) and possess specialized conducting elements. The distal end of the gametangiophore is variously modified in different gametophyte taxa. Antheridia are large and stalked; archegonia are slightly sunken." Taylor et al. (2005).
All homosporous. Intersporangial heterospory (anispory) is typically regarded as an early stage in the evolution of heterospory and is well documented in several groups of fossil plants -- but not found in Rhynie plants. Taylor et al. (2005) "These spore aggregates (Fig. 2P) are known at least for the Rhynie chert sporophytes Aglaophyton and Horneophyton, and we suggest that they may represent a dispersal strategy that resulted in maintaining the close proximity of both types of unisexual gametophytes, thus ensuring fertilization. If Aglaophyton is functionally anisporous, then spore dispersal becomes an important factor in maintaining the next generation, because proximity of sperm and egg is critical." Taylor et al. (2005).
"A slightly different morphology is present in ... the gametophyte of Horneophyton ligneri, and ... the [male] gametophyte of Nothia aphylla. Both ... are ... more complex, with the distal ends ... gametangiophores modified into large cups that contain conducting elements. ... [T]he elaboration of the distal ends of the gametangiophores has little if any phylogenetic significance but rather reflects the physiological complexity of the tips, because it appears that more gametangia are produced per gametangiophore in these gametophytes, compared with those of Aglaophyton�... or even Rhynia�... . Very little is known about ... the development of these two gametophyte types. Early stages in the germination of Horneophyton spores appear similar to those described in Aglaophyton." Taylor et al. (2005).
"sexual expression in these gametophytes was not labile like that in modern homosporous plants. ... the unisexual gametophytes of the Rhynie chert plants suggest that sex determination may occur in the sporangium of the sporophyte " Taylor et al. (2005)
In bryophytes, "Bryophytes also are homosporous but possess a life history in which the sporophyte is reduced and physiologically dependent on the gametophyte, which is the dominant part of the life cycle. Unisexual gametophytes occur in �50% of the taxa (47) and can be directly correlated with spores of two different sizes produced in the same sporangium (48). Some unisexual bryophytes have sex chromosomes, and for several, gametophytes that only produce archegonia are more common (49). Sex expression in bryophytes may be both labile and environmentally controlled (44)," Taylor et al. (2005)
"In vascular plants, the embryo phase is relatively short and the sporophyte soon establishes direct contact with the substrate, thus becoming independent from the gametophyte. Moreover, the sporophyte develops specialized vascular tissues, the xylem and phloem. In particular, the xylem contains waterconducting cells (WCCs), the tracheids and vessel elements, whose developmental pattern includes the deposition of a secondary lignified wall and final cytoplasmic lysis. The bryophytes traditionally include those embryophytes in which the sporophyte is permanently associated with the gametophyte and never establishes direct contact with the substrate." Ligrone et al. (2000).
"Today, the concept of isomorphic early vascular plants is widely accepted." Gerienne et al. (2006).
phytes isomorphic with gametophytes that possess sunken archegonia." Bateman et al. (1998); and multiple sporangia above this level. Kenrick & Crane (1997). That is, "transition to sporophyte dominance moved through a stage in which gametophyte and sporophyte phases were more or less similar in structure (so-called isomorphic alternation of generations." Donoghue (2005) (citing K&C). Contra Boyce (2008). [and Taylor et al. (2005)?]
Stem: "Cooksonia (Figure 10.5) is composed of cylindrical stems that branch in two at various points and which are terminated by cap-shaped sporangia, or spore-bearing structures, at the tip of each branch. The specimens of Cooksonia range from tiny Silurian examples, as little as a few millimetres long, to larger Devonian forms up to 65 mm long." Benton & Harper (1997).
- Small plant attaining heights of 10 cm, dichotomous branched with globose terminal sporangia. Found in monotypic stands. G
The later "rhyniophytes," for example the more derived species of Cooksonia, have steromes, but are still essentially supported by turgor pressure and thus required continuous supply of water. Bateman et al. (1998).
Rhyniophytes with S-type tracheids. Kenrick & Crane (1997a). Not much contribution to mechanical strength. Bateman et al. (1998).
Regardless of whether they have true xylem, these sporophyte axes have intercellular spaces [which Baragwanathia does not], cuticle and stomata [which Silurian Baragwanathia spp. do not], and sporangia. Among those with true xylem are representatives with smooth axes (Rhynia gwynne-vaughanii). Raven & Edwards (2001).
"The vascular conducting tissues of early Devonian examples [of Cooksonia] have thickened walls, and there are stomata on the outer surfaces of the stems." Benton & Harper (1997).
"Water-conducting cells (xylem elements, tracheids) with differentially thickened cell walls are characteristic of the sporophytes of extant vascular plants ... . In nearly all extant vascular plants, conspicuous pits characterize the metaxylem cells, whereas annular or helical thickenings are typical of the earliest protoxylem elements to differentiate (Figure 3.27; Bierhorst 1960). Kenrick & Crane (1997). La présence de xylème chez le fossile Cooksonia du Silurien moyen à supérieur, qui est sans conteste la polysporangiophyte la plus ancienne retrouvée à ce jour (fig. 5), est fortement contestée [peut-être car le lieu phylogénétique de Cooksonia et aussi tant contesté]. Cooksonia partagerait avec d'autres polysporangiophytes du Dévonien inférieur (comme Aglaophyton, fig. 5), la présence de tissus conducteurs non xylémiens proches de ce qui est observé chez les bryopsides. DuBuisson et al. (2005).
S-type tracheids. "The two-layered cell wall comprises a very thin decay-restant inner layer facing cell lumen and a "spongy" outer layer ... . Minute plasmodesmata-sized perforations occur over the entire inner surface of the cell wall." Kenrick & Crane (1997).
Pits allow flow between tracheary elements [equalize pressure, presumably], but allowing air through seeds cavitation and colapse. Sperry (2003). "pitted tracheids evolved independently at least twice (Lycopsida, Euphyllophyta) and that xylem composed entirely of tracheids with helical-annular thickenings is the plesiomorphic condition in vascular plants. Kenrick & Crane (1997: 300)
Water-conducting tissue and stomatal regulation go hand in hand. They appear at essentially the same time in the fossil record and co-occur in the vast majority of extant plants (fig. 1). Sperry (2003). Stomata are characteristic of the sporophyte in basal land plants.
Both rhyniophytes and eutracheophytes had "annular or helical thickenings in the warcr-conducting cells ... .Another possible synapomorpny at this level is lignin deposition on the inner surface of the tracheid cell wall." Kenrick & Crane (1997). "In many early taxa, cells with annular thickenings show occasional diagonal bars connecting adjacent helical-annular rings (e.g., G-type tracheid: Figure 7.27; ... In basal tracheophytes the frequency of diagonal bars is quite low, and their position and number varies such that the cell has a mixture of helical-annular thickenings and simple pits ... We suggest that tracheid pitting evolved through an increase in the regularity and frequency of diagonal bars to produce a more complex pattern of regular pits." Kenrick & Crane (1997: 300-301)
"True tracheids have been defined as single-celled conduits with lignified and ornamented walls, either banded or pitted (Kenrick and Crane 1997a; Doyle 1998). They may have evolved once, uniting all tracheophytes (fig. 1, all taxa below the protracheophytes). The banded type (fig. 3B, 3C) superficially resembles the modern protoxylem tracheid. It is thought to be ancestral because it is the only type found in the basal tracheophyte group, the extinct rhyniopsids. Sperry (2003).
"Cortical sclerenchyma inbasal Euphyllophyta, such as the relatively plesiomorphic Psilophyton, forms a continuous hypodermal band or sterome several cells thick (Figure 7.26). This feature is common in other basal tracheophytes such as the Zosterophyllopsida, Lycopsida, and some Cooksonia- like stem group vascular plants and is probably plesiomorphic in the Euphyllophyta [Eutracheophyta??]" Kenrick & Crane (1997: 297).
Branching still without long-range order in Rhynia, Aglaophyton, Huvenia. Kenrick & Crane (1997). Rhynia and some close relatives show branching from small hemispheres. These vascular strands of these branches are not continuous with the main axis system. Kenrick & Crane (1997). See Fig 1C -- a Silurian form -- in Bodzioch et al. (2003).
Spiny enations common in various rhyniophytes, Psilophyton, Huvenia. Kenrick & Crane (1997).
"Sporangia in Rhynia and Stockmansella are lateral and sessile, whereas in Huvenia they terminate short, branched axes." Kenrick & Crane (1997). "(Stockmansella, Huvenia, Rhynia gwynne-vaughanii), there is a distinctive pad of tissue into which the sporangium is slightly sunken (Figures 4.27 and 4.28). This pad is situated on the end of a short axis or attached laterally on the main axes." Kenrick & Crane (1997). Dehisence through multiple helical slits. Kenrick & Crane (1997).
Gametophyte: "The biological relationship between gametophyte and sporophyte [of Rhynia] is based on the S-type secondary wall thickenings of the conducting elements. The tightly compacted cluster of ... gametophytes (Fig. 2O) consists of �200 globular to bowl-shaped protocorms, each bearing rhizoids on the basal surface and one to several upright unisexual gametangiophores on the distal surface. Of these, 27 are unbranched archegoniophores, and at least 69 additional ones are unbranched antheridiophores. Most gametangiophores grow orthotropically, however, some develop plagiotropically and display a more sinuous growth pattern. This pattern is common in many modern exosporic gametophytes, where crowding is the result of many spores germinating at approximately the same time and becoming intermingled during growth. [Rhynia] antheridiophores are short (≤15 mm) with antheridia located on shield- to cup-like tips; archegonia-bearing axes are longer (≤2.0 cm) with slightly flared tips and archegonia either terminal or subterminal. The morphology and structure of antheridia and archegonia are similar to those of Aglaophyton. Taylor et al. (2005).
"gametophytes from the Rhynie Chert is based on- gametangiophores from three taxa (Remy, Gensel, and Hass 1993). All are small, probably dichotomously branched plants bearing terminal gametangiophores. Anatomically, the gametophyte axes are indistinguishable from those of the associated sporophytes and contain welldeveloped water-conducting tissues as well as an epidermis with stomates." Kenrick & Crane (1997: 302)
"Cladistic analysis indicates that fossils with isomorphic life cycles are related to stem group tracheophytes and probably even to basal Lycophyta (e.g., Nothia aphylla: Table 7.8). This evidence points to a significant elaboration of both the gametophyte and sporophyte in early polysporangiophytes and implies that the gametophytes of extant pteridophytes are highly reduced compared to those of some of the earliest protracheophytes." Kenrick & Crane (1997: 305).
"The specimen [of Cooksonia] is a cluster of five individual sporophytes still attached to the remains of a small female or bisexual gametophyte. In the latter case, this fossil is evidence that reduced thalloid gametophytes and branched axial sporophytes are plesiomorphic among the earliest eutracheophytes. We suggest that a major difference in life cycle defines a basal dichotomy in tracheophytes. Eutracheophyta, including all living vascular plants, have a heteromorphic sporophyte dominant alternation of generations, whereas their extinct sister-group Rhyniopsida (renamed here Paratracheophyta) is characterised by a more or less isomorphic alternations of generations." Gerienne et al. (2006). "implies that the early eutracheophyte Cooksonia had already a heteromorphic sporophyte dominant alternation of generations." Gerienne et al. (2006) Authors acknowledge this is not the only possible interpretation. "According to our Hypothesis # 3, the haploid generation in Cooksonia was much smaller and inconspicuous compared to the diploid phase. Therefore, we propose that Cooksonia had a heteromorphic, diploid dominant, alternation of generations, with axial sporophytes and thalloid prothallus-like gametophytes. This type of life cycle is similar in many respects to that of most freesporing vascular plants living today, and it implies that Cooksonia and all the earliest eutracheophytes had a diplobiontic life cycle with a conspicuous and independent sporophyte. As all previously described Cooksonia specimens (including those of Cooksonia paranensis) consist only of isolated individual sporophytes, it is assumed that the gametophyte/sporophyte association illustrated in the specimen described here is an exception." Gerienne et al. (2006).
"One of the most puzzling nearly whole plants is Cooksonia W. H. Lang, often touted as the earliest vascular land plant but known only from aerial parts (Edwards et al., 1992). Where were the prostrate axes to which these organs presumably were attached? Rothwell (1995) suggested that Cooksonia may have been a sporophyte incapable of a free-living existence, instead growing attached to a photosynthetic gametophyte, much like modern moss sporophytes and, thus, far from completely known morphologically. Gerrienne et al. (2006) found a cluster of Early Devonian Cooksonia axes attached to a thalloid-like pad and offered three possible interpretations, among which was the possibility that the axes represented sporophytes attached to a gametophyte. This hypothesis has been given biomechanical and physiological support by Boyce (2006), who demonstrated that most Cooksonia species are too narrow to have had sufficient photosynthetic tissues to sustain themselves independently." DiMichele & Gastaldo (2008).
Eutracheophytes
Cantino's Tracheophyta. "Synapomorphies. — walls of water-conducting cells with a thick, lignified, decay-resistant layer. Free-living sporophyte and multiple sporangia per sporophyte are synapomorphies relative to other crown clades; however, when fossils are considered, these traits are synapomorphies at a more inclusive level (see /Pan-Tracheophyta). Sterome (a well-developed peripheral zone of the stem consisting of thick-walled, decay-resistant cells) and pitlets in the tracheid wall are listed by Kenrick & Crane (1997: Table 7.2, pp. 11 4, 120) as synapomorphies of “eutracheophytes” (= /tracheophyta), but the extent of missing data for fossils combined with the apparent loss of these traits in extant tracheophytes reduces confidence in their inferred originations." Cantino et al. (2007).
Apical growth and branching coupled with ... were important innovations of vascular plants. In both [eutracheophyte] lineages, however, meristem dormancy and abortion were early innovations, providing evidence of hormonal control and substantial phenotypic flexibility. Kenrick & Crane (1997a). Meloche & Diggle (2001) (explanation of how hormonal control of dormancy and abortion controls branching and phenotypic variability) [Note how this allows overtopping to evolve into true branching by delaying the development of the minor shoot].
Renalia - Longest axis recorded is 11 cm, dichotomous, with round to reniform (kidney-shaped) terminal sporangia. Pseudomonopodial in growth habit of monotypic stands. Displays characteristics of both Rhyniophytes and Zosterophyllophytes. G
Synapomorphies: xylem thickening and loss of columella. Kenrick & Crane (1997). But says that no evidence for columella in Aglaophyton. Kenrick & Crane (1997: 56). So may have evolved earlier. "The eurracheophyte dade (01 = 2) is defined by two structural features of the tracheid eel! wall: a thick, decay-resistant wall, and pitlets between thickenings or within pits (Figure 4.26)." Kenrick & Crane (1997).
"Centrarch maturation of conducting tissues appears to be present in some basal polysporangiophytes such as Nothia [lycopsid by our def and Crane et al. (2004)] and Yunia [stem lycophyte by same criteria] (diarch in part). However, in other taxa, which are often described as centrarch (e.g., Horneophyton, Rhynia), and in other basal polysporangiophytes such as Aglaophyton and Stockmansella, the distinction between protoxylem and metaxylem on the basis of cell size is difficult." Kenrick & Crane (1997). Some had lobes and protoxylem was mesarch in lobes.
"annular or helical thickenings in the water-conducting cells. Another possible synapomorpny at this level is lignin deposition on the inner surface of the tracheid cell wall. ... Include the reduction of sporangium dehiscence to a single well-defined slit and the presence of a sterorne (Figure 4.32. ). Character optimization on our preferred most parsimonious tree (Figure 4.32) also indicate a change in sporangium shape at this node from fusiform and radially symmetrical to elliptical wlth bilateral symmetry. This change is reversed in the Euphyllophyta from node 59 to node 58." Kenrick & Crane (1997).
Dropping CO2 with loss of water use efficiency. Increases cavitation pressure. 17-fold variation Silurian to Pennsylvanian. "Similarly, a given internal storage capacity (pcapacitance) would supply transpired water for 17 times longer under Silurian compared to late Carboniferous conditions. High CO2 conditions would have favored relatively low hydraulic conductance systems with high capacitance." Sperry (2003).
Wood developed in both branches, perhaps independently. Sperry (2003). The additional presence of pitted elements (fig. 3D) resembling metaxylem characterizes most other tracheophyte groups (Kenrick and Crane 1991). Sperry (2003). The lignified secondary walls of tracheary elements provide a significant increase in the compressive strength of the wall—from negligible in nonlignified walls to over 40 MPa (Niklas 1992). The negative pressure required to implode a tubular conduit is a function of the wall thickness divided by the conduit lumen diameter. Sperry (2003). Propping open tracheary elements was probably the original function of lignin and secondary walls in plants (Raven 1987). The thickened and presumably lignified walls of early Devonian vascular plants are limited to tracheary elements of a protostele—a cylindrical strand in the stem center. Sperry (2003). Lignin rigidifies the cell wall in part by replacing water in the cell wall matrix and pore space (Donaldson 2001). In so doing, it reduces the permeability of the walls to water—both by reducing wall porosity and because lignin is hydrophobic. This water-proofing action is not necessary to prevent leakage of water from the conduits as long as the water is under negative pressure, and it has the disadvantage of increasing resistance to water and solute flow across the conduit walls. Sperry (2003).
"The presence of irregular pitlike openings (pitlets) in the cell wall is a common feature in many early polysporangiophyte taxa (Figures 4.25 and 4,26). Such structures are found between annular and helical bars in zosterophylls and basal lycopsids." Kenrick & Crane (1997). And reasonably-well developed xylem known from Silurian Bagwanathia. DuBuisson et al. (2005).
Sterome of similar type in zosterophyls, C. pertoni [stem eutrachophyte], and Psilophyton[same?]; but not Nothia or Asteroxylon [both lycopsids by our def and Crane et al. (2004)]. Kenrick & Crane (1997).
"Biflagellated sperm cells are produced by charophycean algae (except Zygnematales), bryophytes and most [but not all! r et al. note high diversity of types in lycophytes] lycophytes (figures 6 and 7), while all other tracheophytes with motile sperm produce multiflagellated cells (figures 8 and 9). The mature biflagellated cell in bryophytes is a helical cylinder, with an anteriorly positioned locomotory apparatus and four organelles: an anterior mitochondrion, a compacted central nucleus and a posterior plastid with an associated mitochondrion (figure 6b-d). In addition to flagella and basal bodies, the locomotory apparatus consists of a lamellar strip and a narrow band of microtubules (the so-called spline), which extend around the cell providing a framework for positioning of organelles." Renzaglia et al. (2000).
Le cylindre des sporophytes des polysporangiophytes avec ses épidermes verticaux est par contre moins efficace pour capter la lumière. La capture de la lumière peut être favorisée par la sélection de structures horizontales. DuBuisson et al. (2005). la microphylle. Les microphylles vont se généraliser chez les lycophytes et par convergence chez les sphenopsides au Dévonien moyen à supérieur. DuBuisson et al. (2005).
"Extant plants such as horsetails, club mosses, and most ferns are characterized by a haplodiplontic life history in which the sporophyte produces morphologically similar spores (homosporous) that germinate to form free-living, sometimes photosynthetic gametophytes. Sex expression in most is regarded as labile and determined by age and�or presence of an antheridiogen produced by an adjacent gametophyte (44). Slight differences in spore size in homosporous plants have been correlated with stored food, time to germination, and gametophyte development; large spores produce rapidly growing bisexual gametophytes that, in turn, influence smaller spores to produce slower-growing gametophytes with only antheridia. In Equisetum, patterns of sex determination are controlled by various environmental parameters (45). In vitro studies show that crowding, mineral deficiency, high temperatures, drought, and increased sucrose concentrations result in the production of more gametophytes with antheridia. In wild populations, environmental stress appears to have the same effect (46). " Taylor et al. (2005).
"In extant vascular plants, only one sporophyte usually develops, but the presence of multiple sporophytes on one gametophyte is known in extant Sphenophyta, isosporous Lycophyta and Psilotophyta." Gerienne et al. (2006). "On the basis of our Hypothesis # 3, we believe that another characteristic of eutracheophytes might be the presence of a heteromorphic, sporophyte dominant, alternation of generations, while Rhyniopsida would exhibit an alternation of more or less isomorphic generations. Accordingly, we believe that the sets of characters exhibited by the Rhyniopsida and the eutracheophytes respectively differ from each other significantly and therefore we propose the erection of new divisions for the clades: Eutracheophyta and Paratracheophyta. Most fossil vascular plants and all living vascular plants belong to the Eutracheophyta. The oldest known representatives of Eutracheophyta are mid-Silurian (Edwards and Feehan, 1980). Members of the Paratracheophyta (ex Rhyniopsida) include sporophytes (Rhynia, Stockmansella, Huvenia), gametophytes (Remyophyton, Sciadophyton), and morphotypes (Sennicaulis hippocrepiformis, Taeniocrada dubia). Based on current evidence, Paratracheophyta evolved during the early Devonian and became extinct during the late Devonian." Gerienne et al. (2006).
Lycophytes
"The names Lycophyta and Lycopodiophyta have been widely applied to the same set of clades (referring variably to the crown, total clade or something intermediate). Since the former is apparently based on the name Lycopodium, it should be corrected to Lycopodiophyta under the ICBN (Arts. 16.1 and 18.1). The names Lycopsida and Lycopodiopsida are also widely applied to this set of clades" Cantino et al. (2007).
Pan-Lycopodiophyta: "Definition. — The total clade of /Lycopodiophyta. Composition. — /Lycopodiophyta and all extinct plants (e.g., Zosterophyllum) that share more recent ancestry with /Lycopodiophyta than with /Euphyllophyta. ... Synapomorphies. — Possibly sporangium dehiscence by a transverse, apical slit. Doyle (1998) showed this character as arising at or near the base of the (unnamed) lycophyte total clade. Kenrick & Crane (1997: Table 4.6) cited it as a possible synapomorphy of node 52, which is near the base of the total clade" Cantino et al. (2007).
protoxylem generally exarch Kenrick & Crane (1997).
Cambium present but unifacial, with Isoetes the only survivor of this line. Not produce that much wood, since diameter could not increase. "cambial cells in unifacial plants apparently did not divide anticlinally. Consequently, any increases in the cambial ring were brought about by the growth of cambial initials in length, spreading apart the cambial initials situated just above and below them in the cambial cylinder." Donoghue (2005). [show part of figure 6, p.10]
"The Lycophytina are defined by a change from terminal to lateral sporangia (reversed in Hsua)" Kenrick & Crane (1997). "Other synapomorphies appearing in the Lycophytina stem group include (1) isovalvate dehiscence along rhe distal sporangium rim (Figure 4.32, Table 4.6: node 60 -+ node 52), (2) conspicuous cellular thickening of the dehiscence line; (3) reniform sporangia (Figure 4.32, Table 4.6: node S2 -+ node 50), and (4) exarch xylem differentiation (Figure" Kenrick & Crane (1997).
Or toute plante croît d'abord par son extrémité où sont localisées une à plusieurs cellules à forte capacité de division (le méristème). Cette croissance uniquement en longueur se traduit par une limitation dans le diamètre maximal que peut atteindre la tige. Pour croître en épaisseur, l'axe a besoin de tissus supplémentaires. Chez les plantes, la croissance en épaisseur est assurée par la mise en place de méristèmes dits secondaires. DuBuisson et al. (2005). Since lycophytes lack phloem or bifacial cambium, had to grow fat first.
"G-type cell wall with annular-reticulate thickenings typic:al of many zosterophylls and early lycopsids. The two-layered cell wall comprises a decay-resistant inner layer facing cell lumen (white) and a nonresistant outer layer ... ." Kenrick & Crane (1997).
Basal lycophytes of all lineages (Asteroxylon, Drepanophycus, Zosterophyllum) show more evidence of structural support, but most was still supplied by turgor pressure. Bateman et al. (1998).
in the lycophyte line a peculiar bark-like "periderm" tissue (situated in the outer cortex, beneath the persistent leaf bases) was ‘‘invented’’ to stiffen the trunk" Donoghue (2005).
The transition from hydrostatic support to cell-wall support of primary growth was probably first achieved by the evolution of sclerenchyma tissue located peripherally in the stem (Raven 1987; Speck and Vogellehner 1988). Even in the arborescent lycopsids with secondary xylem (e.g., Lepidodendrales; fig. 1), the wood was of limited extent near the stem center, and mechanical support was provided by an expanded cortex and periderm (Gifford and Foster 1989; Kenrick and Crane 1997a). Sperry (2003).
Microphylls developed which are either vascularized or at least influence the growth of axial vascular tissue. Kenrick & Crane (1997). The potential microphyll homologue-the lateral sporangium with a helical sporangiotaxis and a single vascular strand-defines a more inclusive clade that contains zosterophylls and lycopsids. Kenrick & Crane (1997: 291)
"In zosterophylls and lycopsids, sporangia are attached laterally on short stalks (Figures 5.1 and 5.24); in some basal taxa, such as Zosterophyllum myretonianum, Nothia, and Asteroxylon, these stalks are known to he vascular." Kenrick & Crane (1997). Sporangia are symmetrical and isovalvate.
"One variation of the sterilization hypothesis predicts that stem group Lycopsida will have strobilar regions composed of sporangium pairs or sporangia with sporangium-shaped sporophylls on otherwise naked axes. Paired sporangia have not been recorded in the fossil record of this group but would be difficult to recognize in plants with relatively compact strobilate regions. However, sporangium-like sporophylls bearing functional sporangia have been documented in a plesiomorphic member of the zosterophyll-lycopsid clade recently described from China. Adoketophyton subverticillatum bears conspicuous fan-shaped sporophylls, each with a single, adaxial, reniform sporangium on otherwise naked axes" Kenrick & Crane (1997: 291)
Zosterophylls
Gosslingia - Aerial stems up to 0.5 m in height with dichotomous branching but a strong centralized axis resulting in an imperfect pseudomonopodial habit. Small short vascularized branches (<2 mm long) occur along the axis. Commonly found as monotypic assemblages Rebuchia - Plant of densely branched, tufted aspect with distinct upright. G
Sawdonia - Aerial parts up to 30 cm tall (may have attained heights of 0.5 m), pseudomonopodial (anatomically the axes appear dichotomous, but the plant takes on the appearance of a central axis with laterals) from rhizome with lateral dichotomous axes. Apices are coiled (circinate or fiddle-heads such as found in ferns). Lateral spines have no vascular tissue. Reniform (kidney-shaped) sporangia are loosely aggregated into spikes. Commonly found in monotypic assemblages. G.
"Zosterophyllum (Figure 10.5e), a zosterophyllopsid, shares many features with the rhyniopsids, but has numerous lateral sporangia, instead of a single terminal one, on each vertical stem." Benton & Harper (1997).
Zosterophylls had cortical fibers which supplied some mechanical support. Highly developed in derived lycophytes, but lignified core with secondary xylem development required for trees (??) Bateman et al. (1998).
Lack of secondary phloem, limiting transport from photosynthetic areas to growth areas. Consequently, shallow, masive roots with microphylls. Trunk not maintained for long. Donoghue (2005).
Branching may have been planar in basal zosterophylls. Kenrick & Crane (1997). In addition to the main branching system, small subordinate (often undeveloped) axes are common in many zosterophylls. Kenrick & Crane (1997). In zosterophylls, "One common type of branching pattern involves the production of a single, usually small, undeveloped, circinate axis slightly below each dichotomy of the main branching system (Figure 5.15). These small axes are oriented perpendicular to the plane of the main branching system" Kenrick & Crane (1997).
Tree grade independently here, and in both euphyllophyte groups. Donoghue (2005).
Silurian zosterophylls with isotomous branching. Kotyk et al. (2002).
Enations independently acquired in zosterophylls Kenrick & Crane (1997: 291)
"The most likely microphyll homologues occur in the Zosterophyllopsida and closely related taxa" Kenrick & Crane (1997: 290)
Cauline sporangium attachment is the general condition within me Zosterophyllopsida Kenrick & Crane (1997). "Zosterophyllopsida, and these leafless plants bear simple, cauline, lateral sporangia on short, unbranched stalks (Figure 5.1)." Kenrick & Crane (1997).
Lycopsids
Lycopodiophyta: "The least inclusive clade containing Lycopodium clavatum L. 1753, Huperzia selago (L.) Schrank & Mart. 1829, Isoëtes lacustris L. 1753, and Selaginella apoda (L.) Spring 1840." That's the crown clade. For this clade: "association of a single axillary or adaxial sporangium with a sporophyll; absence of vasculature in the sporangium; metaxylem tracheids pitted; root stele bilaterally symmetrical, with phloem located on only one side of the stele (but there are a lot of missing data for fossils outside the crown, so this trait may be synapomorphic of a more inclusive clade); crescent-shaped root xylem (but there are a lot of missing data for fossils outside the crown). The following characters are synapomorphies of this crown clade relative to other crowns but are apomorphic at a more inclusive level when fossils are considered (Kenrick & Crane, 1997: Fig. 6.18, Table 7.2): microphylls (“lycophylls”; Schneider & al., 2002; Pryer & al., 2004a); exarch xylem differentiation in stem (Kenrick & Crane, 1997; Doyle, 1998; Schneider & al., 2002); stellate xylem strand in stem; reniform sporangia with transverse dehiscence (Doyle, 1998). This list is not exhaustive; see Kenrick & Crane (1997: Table 7.2) and DiMichele & Bateman (1996) for other synapomorphies listed under Lycophytina and Lycopsida." Cantino et al. (2007).
Nothia - Naked invaginated axes with pear-shaped sporangia (apical dehiscence) on adaxially (downward) recurved stalks. Sporangia may be helical or whorled in arrangement. Found with other rhyniophytes. G
"[Rhynie Chert] sporophytes have branched aerial axes (i.e. are polysporangiophytes: Kenrick and Crane, 1997) which are endohydric, but do not always have true xylem (i.e. water conducting cells with unevenly but regularly thickened walls typical of tracheids); an example of an endohydric plant without true xylem is Nothia aphylla
Asteroxylon attained a height of 0.5 meters and consisted of erect monopodial branches originating from a flat-lying (prostrate) rhizome. The xylem cylinder is an actinostele (star-shaped). Axes are covered with "enations" (appear to be leaves but lack vascular tissue) with stomata. Kidney-shaped sporangia (reniform) are borne in the axil of an enation. Vascular tissue has been identified in the sporangial area. G.
Asteroxylon with microphylls. Raven & Edwards (2001). The leaf of the early fossillycopsid Asteroxylon is often cited as evidence favoring the enation hypothesis because of the leaf's enation- like morphology. Unlike in other lycopsids, the leaf trace of the fossil terminates at the base of the leaf. Asteroxylon is unique in this respect, but it is equally parsimonious to interpret the absence of vasculature within the leaf as a loss. Kenrick & Crane (1997: 290). Microphylls independently acquired in Lycopsida. Kenrick & Crane (1997: 290-291)
"lycophyte Baragwanathia, cuticle, stomata, tracheids, and (probably) intercellular gas spaces are known from above-ground structures in Lower Devonian specimens (but not so far from the less well preserved Upper Silurian fossils) (Hueber, 1983), Raven & Edwards (2001).
"anatomical studies of arborescent lycopsids raise certain matters with regard to their physiology (Phillips & DiMichele, 1992). The lack of a clear phloem connection between root and shoot, the generally limited phloem throughout the plant’s aerial shoot, the leaf-like rootlets borne on the stigmarian axes, and the long leaves on stems and cones, are all consistent with extremely localized use of photosynthate and perhaps even self-supporting root systems in terms of carbon fixation." DiMichele & Gastaldo (2008).
"In lycopsids, antheridia are embedded individually within the tissues of the gametophyte, whereas antheridia in other vascular plants, such as the Ophioglossaceae, Equisetaceae, Psilotaceae, and leptosporangiate ferns, are sessile on the surface of the gametophyte." Kenrick & Crane (1997).
Wood developed early, but fairly deep withing the lycopsid lineage (Lepidodendrales) Sperry (2003).
"secondary vascular tissues evolved independently in at least two major groups of vascular plants. These two lineages of secondary vascular tissues were not homologous (Cichan, 1985). ... Species of the second group (or groups) produced either a unifacial vascular cambium or a cambium that differentiated secondary vascular tissues only toward the inside, and except for the quillwort Isoetes (Lycopsida), are all extinct" Rothwell & Lev-Yadun (2005).
The association of a single sporangium with a sporophyll is a unique feature of lycopsids ... Usually, the sporangium is axillary to the sporophyll or positioned on the basal part of its adaxial surface, but in some taxa it is well out on the leaf. ... In certain early fossils, such as Asteroxylon ... and Drepanophycus ... sporangia clearly are cauline and do not appear to be associated with specific "sporophylls." but in others. such as Baragwanathia, the condition is unclear ... . It is uncertain whether the cauline sporangia of such plants as Asteroxylon are loosely associated with a leaf or completely dissociated from leaf phyllotaxy (Kenrick & Crane, 1997: 201). [not certain this is consistent with k&C:289]
"Three hypotheses concerning the origin of the lycopsid microphyll focusing on the sporophyll-sporangium appendage (transitions occurring from left to right). (a) The reduction hypothesis interprets the sporangium-sporophyll appendage in lycopsids as a highly reduced lateral branch. Sporophylls of putative extinct intermediates are characterized by branched "leaves" and multiple sporangia. Modified after Stewart and Rothwell 1993. (b) The enation hypothesis interprets the sporophyll as a new structure that evolved as a sterile outgrowth of the stem. Sporophylls of putative extinct intermediates are characterized by unbranched and nonvascular or partially vascular "leaves" (path of vascular tissue indicated within stem). [as K&C note, these fail to explain association of sporophyll with leaf] (c, d) The sterilization hypothesis interprets the sporophyll as a sterilized sporangium. Sporophylls of putative extinct intermediates are characterized by unbranched and partially vascularized leaves that may be reniform or spatulate in shape (path of vascular tissue indicated within stem). In (c), the sporangiumsporophyll association arises by sporangium duplication prior to sterilization. In (d), the sporangium-sporophyll association arises by subsequent association of sporangium and microphyll." Kenrick & Crane (1997: 289)
We suggest that the lycopsid microphyll is a transformational homologue of the sporangium.... Sporangium and sporophyll share similar positions on the stem, and the expression of this positional similarity is continued in the phyllotaxis of vegetative leaves. Both organs also have a similar ontogeny, developing as lateral structures from epidermal initials close to the stem apex.... Similarity of sporangium vascularization among early members of the Lycophytina to vascularization of microphylls in Lycopsida is also significant. Kenrick & Crane (1997:291)
In Asteroxylon, both microphylls and sporangia possess their own vascular trace. The absence of vasculature within the microphyll of Asteroxylon is consistent with the absence of vasculature in equivalent areas of the sporangium. Kenrick & Crane (1997: 291)
"During gametophyte ontogeny in the Lycopodiaceae, apical growth ceases at a very small size, and the peripheral cells on the apical flanks become meristematic, forming a ring meristem. Subsequent cell divisions in the ring meristem produce an expanded, disk-shaped apex that is typically 3- 15 mm in diameter and has a smooth or convoluted margin (Figure 7.28). The archegonia and antheridia are located on the upper surface of the disk." Kenrick & Crane (1997: 305) The disk-shaped gametangiophores in the permineralized Rhynie Chert plant Kidstonophyton (sporophyte = Nothia aphylla) and in the compression fossils of the Sciadophyton type strongly resemble gametangiophore morphology in extant Lycopodiaceae (Figure 7.28). Both Kidstonophyton and Lycopodium clavatum gametangiophores comprise an expanded, disk-shaped structure of similar size and shape. Both bear gametangia on the convex upper surface and have a raised marginal rim without gametangia. Kenrick & Crane (1997: 305)
"It is worth pointing out that the gametophyte of Lycopodium is perennial, and may persist at the base of the mature sporophyte (Raven et al., 1999)." Gerienne et al. (2006).
Stem Euphyllophytes
Euphyllophyta crown group: "The most inclusive crown clade containing Ginkgo biloba L. 1771 (/Spermatophyta), Equisetum telmateia Ehrh. 1783, and Pteridium aquilinum (L.) Kuhn 1879 (/Leptosporangiatae) but not Selaginella apoda (L.) Spring 1840 (/Lycopodiophyta)." ... "Synapomorphies (relative to other crown clades). — ... sporangia terminating lateral branches (Pryer & al., 2004a) and dehiscing longitudinally (Doyle, 1998) (these features characterize the earliest members of /Pan-Euphyllophyta and were modified in most extant representatives); lobed, mesarch primary xylem strand (Stein, 1993; Kenrick & Crane, 1997: Fig. 7.10 and p. 241; Doyle, 1998), which has been modified in the stems of most extant members; multiflagellate spermatozoids (apparently convergent in Isoëtes) (Garbary & al., 1993; Kenrick & Crane, 1997: 240, 275); a 30-kb inversion in the chloroplast genome (Raubeson & Jansen, 1992a). Megaphylls (euphylls) are sometimes cited as a synapomorphy of /Euphyllophyta (Schneider & al., 2002), but analyses that include fossils suggest that the compound, fernlike megaphylls of monilophytes and seed plants evolved independently (Stewart & Rothwell, 1993; Kenrick & Crane, 1997; Doyle, 1998; Boyce & Knoll, 2002; Friedman & al., 2004). Even within /Lignophyta, the small, wedge-shaped leaves of Archaeopteris may not be homologous with the whole fernlike fronds of seed ferns but rather with individual leaflets of such fronds (Doyle & Donoghue, 1986a; Doyle, 1998)." Cantino et al. (2007).
Pan-Euphyllophyta: "Euphyllophyta and all extinct plants (e.g., Psilophyton) that share more recent ancestry with /Euphyllophyta than with /Lycopodiophyta. Synapomorphies. — Several synapomorphies were listed by Kenrick & Crane (1997: 240, Table 7.2, and pages listed below), most of which have been lost or modified in some or all extant members of the clade: pseudomonopodial or monopodial branching (pp. 109, 359) (although if the fernlike leaves of early seed plants were derived from pseudomonopodial branch systems of more basal lignophytes (Doyle, 1998), the axillary monopodial branching of seed plants and the pseudomonopodial branching of more basal lignophytes may not be homologous); helical arrangement of branches (pp. 11 0, 360); dichotomous appendages (pp. 11 3, 361); recurvation of branch apexes (pp. 11 2–11 3, 360); paired sporangia grouped into terminal trusses (pp. 121–122, 364); sporangium dehiscence along one side through a single longitudinal slit (pp. 125, 366). Kenrick & Crane also cited scalariform bordered pitting of metaxylem cells as a synapomorphy, but it does not occur in Eophyllophyton and therefore is synapomorphic for a slightly less inclusive group than the total clade (op. cit., pp. 120, 363, Fig. 7.10)." Cantino et al. (2007).
Psilophyton - Variable branching of the erect stem (pseudomonopodial, dichotomous or trichotomous) that emerged from rhizomatous mat. Stem with variously shaped spines. Paired sporangia terminate ultimate dichotomy of the lateral branches; homosporous. Central axis may be as much as 3.5 cm in diameter; maximum height may have been > 1m. G.
Pertica - Lateral branches are tetrastichous (four branches in an opposite pattern), forming a clockwise spiral, and originate from a main axis Individual branches dichotomize many times at right angles to each other. Axes have small bumps (papillae). Clusters of sporangia (32-256) terminate branches; trilete spores are homosporous. Main axis 1.5 cm in diameter; maximum height may have been several meters. G
"The Euphyllophyta are supported as a group by six synapomorphies: (1) pseudomonopodial or monopodial branching, (2) a basically helical arrangement of branches, (3) small, "pinnulelike" vegetative branches (nonplanare in basal taxa), (4) recurvation of branch apexes, (5) sporangia in pairs grouped into terminal trusses, and (6) multicellular appendages (spines)." "Other synapomorphies appearing in this stem group indude (1) meta xylem pitting, (2) aligned xylem, (3) radial sporangium symmetry (reversal), (4) fusiform sporangia (reversal) (Figure 4.32, Table 4.6: node 59 ~ node 58), (51 the loss of multicellular spines (reversal) (Figure 4.32, Table 4.6: node 58 ~ node 57), (6) a lobed xylem strand, and (7) a change from 3 continuous sterome to discrete hundles of fibers in the stem correx (Figure 4.32, Table 4.6: node 57 4 node 56). This result supports the independem origins of pseudomonopodial branching, meta xylem pitting, and lohed xylem strands in the lycophytina and toc Euphyllophyta." Kenrick & Crane (1997).
True tracheids in basal euphyllophytes. Donoghue (2005).
Protoxylem generally mesarch to centrarch. Kenrick & Crane (1997).
"P-type cell with scalariform pitting typical of plesiomorphic Euphyllophyta (e.g., Psilophyton). The two-layered cell wall comprises a decay-resistant inner layer facing the cell lumen, pit chambers, and a nonresistunt inner layer within the scalariform bars ... . Characteristically, the scalariform pit apertures are covered with a perforate sheet of decay-resistant material." Kenrick & Crane (1997).
"overtopping (or pseudomonopodial growth) evolved at the base of the euphyllophytes" possibly in multiple lineages. Enabled the evolution of megaphyllous leaves. Branching equivalent to development of hox polarity in animals and may involve similar genes (i.e. knox). Donoghue (2005). Computer simulations suggest that overtopping preceded planation, which preceded webbing. Niklas (2004). Interestingly, Niklas notes this occurs on the assumption that light interception and mechanical stability are the main criteria (i.e. not spore dispersal or water conservation). He does not say what happens when these are factored in or substituted. His series reproduces Psilophyton wonderfully from a very basal Cooksonia. It does not reproduce lycophytes or Archaeopteris. Suggests that other factors (competition? predation?) were more significantly limiting than light and mechanical stability in those cases.
"basal eupbyllophytes many taxa exhibit a radial alignment of the metaxylem, but there is often no histological evidence of cambial activity, and ray cells are absent." However, "Radially aligned xylem, whether or not the product of cambial activity, has been noted in tbe larger axes of [some species of] Psilopbyton." Kenrick & Crane (1997). Psilophyton shows aborted branches, although quite different from zosterophylls. Kenrick & Crane (1997).
But branch structure in Psilophyton like the simplest axes of Aglaophyton. Kenrick & Crane (1997). Branch pattern was helical. Kenrick & Crane (1997). Development of various kinds of long-range order in lineages.
"In ferns and seed plants, much morphological diversity is clearly attributable to modifications of branching systems into a variety of leaf-like organs, whereas the relatively conservative clubmoss bauplan has a dearth of organ systems that can be interpreted as modified branches." Kenrick & Crane (1997a). But see Kotyk et al". (2002).
"Much of the morphological diversity in the Euphyllophyta seems attributable to profound modifications to the branching systems, whereas morphological conservatism within the Lycophytina is reflected in the absence of elaborate branching and the dearth of organ systems that can be interpreted as transformed branches." Kenrick & Crane (1997: 298)
Leaves appeared as a result of falling carbon dioxide concentrations. Prior to Mid-Devonian, expanses of photosynthetic area would have killed the plant from heat stress. Beerling et al. (2001). Associated with 100x increase in density of stomata. Earliest leaves in Eophyllophyton. See also image here.
"Isotomously branching, nonplanar, small, ultimate appendages are present in many taxa of the Euphyllophyta. These structures are leaflike in several respects but are unwebbed." Includes Psilophyton. Kenrick & Crane (1997). Les euphyllophytes sont caractérisées par un mode de croissance particulier qui se traduit par une latéralisation des rameaux, c'est-à-dire qu'on l'on a un axe principal vertical et des ramifications latérales (elles-mêmes ramifiées) de diamètre plus petit que celui de l'axe principal (ce qui s'observe dès le Dévonien inférieur chez Psilophyton). DuBuisson et al. (2005). La vraie feuille ou mégaphylle serait une ramification latérale transformée avec mise dans un même plan horizontal de toutes les ramifications du rameau et développement d'un limbe (le tissu aplati qui constitue les feuilles) réunissant ces ramifications. L'apparition de cette feuille est néanmoins relativement tardive, au Dévonien supérieur, par convergence chez les filicopsides et chez les premières gymnospermes. DuBuisson et al. (2005).
"The enation hypothesis predicts that the microphyll character will be nested within a more general clade defined by the presence of enations. Although nonvascular enations are common in many early land plants, this feature is highly homoplastic (see Chapter 3). Bower (1935) used the enations of Psilophyton princeps as a model for an intermediate stage in microphyll evolution, but our analysis shows that enations in Psilophyton evolved independently from those in the Lycophytina" Kenrick & Crane (1997). Really? See "Multicellular spines are common in many zosterophylls, where they may be filiform ... or deltoid in shape ... . Morphologically similar spines are found in some rhyniophytes, such as Huvenia and Dutoitea, and also in some species of Psilophyton ... . Microphylls of basal homosporous lycopsids generally are somewhat larger than the spines discussed above and are either fully vascularized or clearly influence the differentiation of vascular tissue in the adjacent axis (e.g., Asteroxylon: Figure 6.16). We define the microphyll as a stem outgrowth that influences the differentiation of axial vascular tissue." Kenrick & Crane (1997: 113) Enation hypothesis still possible. K&C favor idea that "all the appendicular structures of lycopsids (microphylls, ligules, sporangia) as iterative modifications of a single basic developmental pathway (Figure 7.21). We suggest that the lycopsid microphyll is a transformational homologue of the sporangium." Kenrick & Crane (1997: 291)
Our preliminary c1adogram for the Euphyllophyta supports a hypothesis of homology among megaphylls in seed plants, ferns, and Equisetum (highly reduced) at the level of dichotomous, threedimensional lateral branches of the Psilophyton type. This result is consistent with the independent evolution of planation, webbing, and fusion in the major clades of megaphyllous plants. Thus, many of the similarities between megaphylls in ferns and in basal seed plants are convergent. Probably only the open dichotomous vasculature typical of many basal members of these clades can be viewed as homologous. Kenrick & Crane (1997: 295). This consistent with the hypothesis of forcing by lowered CO2, increased stomata. Osborne et al. (2004).
"In trimerophytes, progymnosperms., and many early fernlike taxa [including Psilophyton], sporangia are paired in densely hranched clusters at the ends of smaller branches." Kenrick & Crane (1997). Dehisence in Psilophyton, Pertica seems to be from a single slit on one side of the sporangium. Kenrick & Crane (1997).
Sperm are larger, all multiflagellate, with more abundant organelles (suggesting bigger investment in sperm durability and mobility). Also, "Cellular elongation or streamlining reduces excess baggage and, perhaps even more importantly, is instrumental in movement of the spermatozoid through the narrow tube of the archegonial neck." Renzaglia et al. (2000).
. "pteridophytes, in which the single epidermal initial first divides transversely (periclinal division). The outer cell then further divides to form the neck while the inner cell divides periclinally to produce neck canal cells, ventral canal cell and egg (figure 2f, g, j) (Bierhorst 1971; Kenrick & Crane 1997a). With expansion, the archegonium projects from the epidermal surface. The neck invariably consists of four vertical rows of cells (figure 2h, i). Clearly, there are no parallels in development between the sunken archegonia of hornworts (figure 2e) and similar embedded archegonia in pteridophytes (figure 2j)." Renzaglia et al. (2000).
Monilophyta
Unifacial cambium. Donoghue Tree habit through support from roots or intertwining of numerous small stems. (2005).
Some developed wood, but (per fossil record) not until Jurassic. Sperry (2003).
"Synapomorphies (relative to other crown clades). — A possible synapomorphy is the exclusively centrifugal development of the spore exine (Schneider & al., 2002)" Cantino et al. (2007) This for the crown group -- not for Pan-Monilophyta which is actually Cantino et al.'s equivalent of this clade. Definition is the total clade of "the most inclusive crown clade containing Equisetum telmateia Ehrh. 1783 and Pteridium aquilinum (L.) Kuhn 1879 (/Leptosporangiatae) but not Ginkgo biloba L. 1771 (/Spermatophyta) or Selaginella apoda (L.) Spring 1840 (/Lycopodiophyta)." Cantino et al. (2007).
Modern Equisetum may be viewed as an example of an analogous stage in the evolution of the breeding system that we hypothesize in the Rhynie chert plants. Equisetum spores contain a pair of hygroscopic elaters that function in dispersing spores from the sporangium. However, these appendages also become entangled with those of other spores so that clusters of spores are dispersed together. This event results in the development of multiple gametophytes in close juxtaposition and optimizes the opportunity for fertilization and the development of new sporophytes while reducing the chances for outbreeding. Gametophytes of Equisetum may be photosynthetic and long-lived and may produce either antheridia or archegonia initially; gametophytes with archegonia eventually develop antheridia, whereas those with antheridia rarely produce archegoniaTaylor et al. (2005).
Lignophytes
When developed in early lignophytes, originally limited to main axis. Id.
Wood common to all Sperry (2003)
Trimerophytes had P-type. Kenrick & Crane (1997a).
Bifacial cambium developed by end of Devonian. "Cambial cells in lignophytes can undergo both periclinal and anticlinal cell divisions, the periclinal ones adding xylem and phloem and the anticlinal ones adding extra cells to the ring of cambium. Donoghue (2005). Un premier méristème se forme entre les phloème et xylème primaires, c'est le cambium et il produit au moins un nouveau xylème dit secondaire qui croît radialement. Ce nouveau xylème est le bois que l'on trouve dans les troncs. Un deuxième méristème (nommé phellogène) se forme vers la périphérie de la tige et donnera l'écorce. La présence d'un bois et d'un tronc définit l'arborescence, et donc l'arbre, et autorise de très grandes tailles, via non seulement l'augmentation du diamètre mais aussi via l'ajout de nouveaux tissus lignifiés plus ou moins rigides. DuBuisson et al. (2005). Developed fully by Archaeopteris. DuBuisson et al. (2005) (fig 7).
"secondary vascular tissues evolved independently in at least two major groups of vascular plants. These two lineages of secondary vascular tissues were not homologous (Cichan, 1985). The first was in a clade that includes the extinct progymnosperms (Crane, 1985; Rothwell and Serbet, 1994) and seed plants, where a bifacial vascular cambium usually forms xylem toward the inside and phloem toward the outside (Larson, 1994)." Rothwell & Lev-Yadun (2005)
"When various obstacles such as buds, branches, and wounds disrupt the axial polar auxin flow in or near the cambial region of seed plants (conifers and dicotyledons), polar auxin flow whirlpools are formed in the cambial zone (Sachs and Cohen, 1982). These auxin whirlpools induce the differentiation of characteristic circular tissue patterns of tracheary elements above axillary buds of woody plants ... and at branch junctions in the wood of conifers (Lev-Yadun and Aloni, 1990) (Fig. 2) and dicotyledonous woody plants (Lev-Yadun and Aloni, 1990) (Fig. 3). Identical circular patterns also occur at the same positions in the secondary wood of the Upper Devonian fossil progymnosperm Archaeopteris (Figs. 4, 5), thus providing the first clear fossil evidence of polar auxin flow. Such spiral patterns do not occur in the primary xylem of either fossil progymnosperms or extant seed plants. They are also absent from the primary xylem strands of living ferns and other nonseed plants such as Psilotum nudum (L.) Beauv., and from the Lower Devonian fossil land plants that we have examined [ca. 390-385 million-years-old Aglaophyton major (Kidston and Lang) D. Edwards; Renallia hueberi Gensel; and Psilophyton dawsonii Banks, Leclercq & Hueber]." Rothwell & Lev-Yadun (2005).
Reproduction
"enlargement and branching of the sporophyte preceded the acquisition of tracheids" Donoghue (2005).
Included in the rhyniophytes are ancestral forms are the ancestral genus Cooksonia, from the late Silurian to Late Devonian (Wenlock to Frasnian). By the Early Devonian more advanced types like Rhynia had evolved.
delayed initiation of spore-bearing organs Kenrick & Crane (1997a). Note zosterophylls with spores on retarded branches approaching a Pseudomono condition. Kotyk et al. (2002).
Ecology
 "The evolution of roots is thought to have been an important factor in the reduction of atmospheric CO2 concentrations through increased weathering of Ca–Mg silicate minerals brought about by mechanical disruption and soil acidification." Kenrick & Crane (1997a). Raven & Edwards (2001).
"The evolution of roots is thought to have been an important factor in the reduction of atmospheric CO2 concentrations through increased weathering of Ca–Mg silicate minerals brought about by mechanical disruption and soil acidification." Kenrick & Crane (1997a). Raven & Edwards (2001).
"the maximum observed aerial axis diameter of vascular plant fossils from the late Silurian to the Devonian-Carboniferous boundary [increases] from some 3 mm diameter in the latest Silurian, via an approximately linear increase in the logarithm of diameter with time, to almost 2 m at the end of the Devonian in the progymnosperm Archaeopteris (Callixylon). [Theory] suggest a height for Archaeopteris of 10-30 m. Corresponding to this increase in height is an increased plant biomass per unit land area and depth of penetration of roots sensu lato. ... there is independent evidence of an increasing depth of penetration of roots ... up to 2 mm in diameter and up to 0.9 m long in the Emsian (late Early Devonian) have been found." Raven & Edwards (2001). Uncertain effects, but presumably large. R&E miss the point that the change of cycling could have been way out of balance.
Siluro-devonian increase in oxygen and decrease in CO2. But see Scott & Glasspool (2006).
Herbivory by Middle Devonian? Bateman et al. (1998).
least one form, Taeniocrada is thought to have been aquatic, although whether transitional forms from fresh water to land or secondarily aquatic is not clear.
Expansion into an empty ecospace, with rapid radiations in a "fractal" pattern. Bateman et al. (1998).
Notes
[1] These generalizations lose much of their force over the course of the Mesozoic. We are speaking only of the Paleozoic pattern. A Paleozoic naturalist would rarely find 100 temnospondyls of the same species in one place, but -- if he did -- he could count on each one having exactly the same number of limbs. For Paleozoic plants, the case would be exactly the reverse.
uploaded ATW050928
modified ATW090426
 We have reorganized this section several times, generally ocillating between phylogenetic anatomy and anatomical phylogeny. We ultimately reached the conclusion that there is no reasonable way to organize this material. We suspect that Kenrick & Crane (1997) found the same problem a dozen years ago, since they ultimately describe the anatomy of early plants several times, using various different schemes of organization. In fact, we strongly recommend Kenrick & Crane (1997) to the interested reader. The review below is written -- as is most of Palaeos -- solely for the purpose of educating ourselves. For that purpose, we will take an anatomical approach, trying to make sense of the evolution of the various organ types using the consensus phylogeny. Kenrick & Crane (1997), who invented the now consensus phylogeny, do this far better than we could ever hope to do.
We have reorganized this section several times, generally ocillating between phylogenetic anatomy and anatomical phylogeny. We ultimately reached the conclusion that there is no reasonable way to organize this material. We suspect that Kenrick & Crane (1997) found the same problem a dozen years ago, since they ultimately describe the anatomy of early plants several times, using various different schemes of organization. In fact, we strongly recommend Kenrick & Crane (1997) to the interested reader. The review below is written -- as is most of Palaeos -- solely for the purpose of educating ourselves. For that purpose, we will take an anatomical approach, trying to make sense of the evolution of the various organ types using the consensus phylogeny. Kenrick & Crane (1997), who invented the now consensus phylogeny, do this far better than we could ever hope to do.  To decide how roots evolved, we must decide what
To decide how roots evolved, we must decide what 
 "The evolution of roots is thought to have been an important factor in the reduction of atmospheric CO2 concentrations through increased weathering of Ca–Mg silicate minerals brought about by mechanical disruption and soil acidification."
"The evolution of roots is thought to have been an important factor in the reduction of atmospheric CO2 concentrations through increased weathering of Ca–Mg silicate minerals brought about by mechanical disruption and soil acidification."