
| Palaeos: |  |
Demospongiae |
| Porifera | Demospongiae - 3 |
| Page Back | Unit Back | Metazoa | Metazoa Dendrogram | Metazoa References | Pieces | Taxon Index |
| Page Next | Unit Next | Unit Home | Unit Dendrogram | Unit References | Glossary | Time |
PORIFERA |--Archaeocyatha `--+--Stromatoporoidea `--+--Calcarea `--+--Hexactinellida `--+--Demospongiae | |--Tetractinomorpha | `--Ceractinomorpha `--+--Homoscleromorpha `--EUMETAZOA |
Introduction Spicules: a New Look Sponge Skin Rootlets and Relationships New Developments History The Sequence of Sponges
|
I was present at an undersea, unexplained
mass sponge migration.
--Ghostbusters (1984)
Actually the matters we are not going to cover here include motility and photosensitivity. These are interesting topics, but have little phylogenetic potential. Slow motility, in the mm/day range, seems to be common to both calcareous and siliceous encrusting sponges. Not a great deal is known about this phenomenon in any sponge.
Larval photosensitivity is more promising. Some calcareous sponge larvae have exactly four "cruciform cells" evenly distributed about their equator. It has been speculated that these cells are photosensitive, but no phototaxis has been observed. Demosponge larvae, by contrast, have a very definite negative phototactic response. Light must be a traumatic experience for young sponges, as it causes their cilia to stand straight up on end. This reaction seems to be related to pigment located in the cell bodies of a subequatorial ring of cells with elongate cilia. A well-developed reaction to light thus may be unique to demosponge larvae. However, we have very little comparative data, particularly outside the demosponges. Leys & Degnan (2001); Leys et al. (2002); c.f. Amano & Hori (2001).
 What we are going to discuss is sponge development. As we have
just seen, sponges avoid the bright lights and don't run around much. They
don't wear a lot of make-up. They settle down early, and usually get pregnant in a
discreet manner -- so discreet that in many cases the male gamete has never been
observed. It may seem peculiar that sponges should get pregnant and
bear live young (Leys
& Ereskovsky, 2006). But, then, we've always felt that way about English
majors, too. Somehow, one feels that they ought lay large, intricately
colored eggs, like a
Fabergé kiwi. In fact, some sponges are
oviparous. Most or
all of these oviparous sponges are also demosponges, but none are English majors
-- although it may sometimes be difficult to make these fine distinctions by casual
inspection.
What we are going to discuss is sponge development. As we have
just seen, sponges avoid the bright lights and don't run around much. They
don't wear a lot of make-up. They settle down early, and usually get pregnant in a
discreet manner -- so discreet that in many cases the male gamete has never been
observed. It may seem peculiar that sponges should get pregnant and
bear live young (Leys
& Ereskovsky, 2006). But, then, we've always felt that way about English
majors, too. Somehow, one feels that they ought lay large, intricately
colored eggs, like a
Fabergé kiwi. In fact, some sponges are
oviparous. Most or
all of these oviparous sponges are also demosponges, but none are English majors
-- although it may sometimes be difficult to make these fine distinctions by casual
inspection.
This is only the first of many demosponge developmental peculiarities. Sponges in general have more developmental diversity than any other group of animals, bar none. For example, embryologists have been debating the existence and nature of gastrulation in sponges since Haeckel's time, despite the fact that Haeckel based the concept of gastrulation on the development of calcareous sponges. Not only do sponges differentiate "inside" and "outside" in a wild variety of different ways, but no one is absolutely sure which, if any, of these moves is actually homologous to gastrulation in bilaterians.
Perhaps we should approach this subject a little more systematically. Sponge gametes are generally formed from the choanocyte cell lineage, with some exceptions. Leys & Ereskovsky (2006). Some maternal tissue is usually released with the embryo, and even accompany the egg in oviparous forms. In addition, each embryo takes along a selection of maternal bacteria, like a folder of family recipes. While immaterial to the present discussion, note that this presents both theoretical and practical problems. The average sponge newborn has a good deal of functional DNA hanging around, bacterial and maternal, which is not part of its own genome.
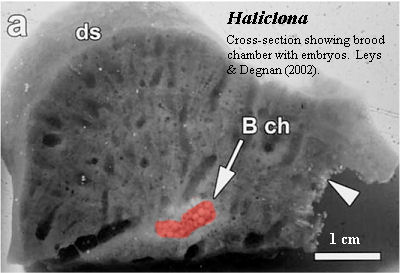
The usual demosponge larva is referred to as a parenchymella. Like sponge larvae generally, it has a distinct outer "epithelial" layer including small ciliated cells (micromeres) and a distinct front and back end. Whatever the sponge adult may look like, the sponge larva is always radially symmetrical. Development of the parenchymella larva of Haliclona (= Reniera) is very nicely summarized by Larroux et al. (2006). The following is essentially a quotation, but patched together from two separate parts of their paper. In addition, we have omitted citations and some express comparisons with metazoan embryogenesis.
The haplosclerid demosponge Reniera [Haliclona] broods embryos throughout the year. After fertilization there is a period of cell division with little or no cell growth. Cleavage ends with an asymmetric cell division, which gives rise to two cell populations -- micromeres and macromeres -- which are initially mixed with each other. This is followed by a period of cell sorting that produces an embryo consisting of inner macromeres and outer micromeres. At this stage a number of differentiating cell types are evident in the micromere population, specifically a large group of uniciliated cells, and smaller groups of pigmented cells and spicule-producing sclerocytes. Once on the surface, pigment cells and some sclerocytes begin migrating to the future posterior pole of the larva in a predictable manner. The pigment cells form an external ring that surrounds the posterior pole. The sclerocytes ingress [generally into the posterior half of the] inner cell mass. At this stage, a middle cell layer also forms. Embryogenesis culminates in a swimming larva composed of at least 11 cell types, each of which is allocated to a different cell layer and in some cases patterned along the AP axis, which is defined by the direction of larval swimming and can be readily identified by the posterior location of the pigment ring. At metamorphosis the larva undergoes dramatic changes that include the loss of overt body axes, the migration and transdifferentiation of specific cell types and the formation of choanocyte chambers
 It is important to notice that all this shuffling of micromeres differs from
histogenesis in most Eumetazoa. The micromeres are initially mixed
in with everything else and seem to migrate as individual cells, not as a
tissue. On the other hand, we know very little about cell-cell
communications in sponges. We can't really tell whether these are free-market,
Objectivist micromeres, influenced only by self-actualizing responses to an
Invisible Hand, or,
instead, class-conscious
Socialist
micromeres, marching forward to their destiny in histological solidarity. Cell
fate does seem to be determined early, before migration, but even that isn't
really clear. All we can say for certain is that sponges rarely practice
histogenesis by differentiation of a particular mass of contiguous cells.
Degnan et al.
(2005); Larroux et al. (2006); Leys & Ereskovsky (2006). At the same time,
the variable, and often chaotic, pattern of cleavage in the late blastula stage
of many sponges also means that the blastomeres are not chained to their fate
until at least the end of blastula. Degnan et al. (2005).
It is important to notice that all this shuffling of micromeres differs from
histogenesis in most Eumetazoa. The micromeres are initially mixed
in with everything else and seem to migrate as individual cells, not as a
tissue. On the other hand, we know very little about cell-cell
communications in sponges. We can't really tell whether these are free-market,
Objectivist micromeres, influenced only by self-actualizing responses to an
Invisible Hand, or,
instead, class-conscious
Socialist
micromeres, marching forward to their destiny in histological solidarity. Cell
fate does seem to be determined early, before migration, but even that isn't
really clear. All we can say for certain is that sponges rarely practice
histogenesis by differentiation of a particular mass of contiguous cells.
Degnan et al.
(2005); Larroux et al. (2006); Leys & Ereskovsky (2006). At the same time,
the variable, and often chaotic, pattern of cleavage in the late blastula stage
of many sponges also means that the blastomeres are not chained to their fate
until at least the end of blastula. Degnan et al. (2005).
We have barely mentioned gastrulation, because, in respect of sponges, the mere utterance of that word has the same effect as a prefrontal lobotomy, shutting down volition, analysis and imagination. Thus, some worker sees three tissue layers, another utters the G-Word, and all nod vaguely. Then, for a minute or two, everyone peers vaguely into space, as if anaesthetized. Finally, discussion abruptly takes off again, on some completely unrelated tack. It's really quite peculiar.
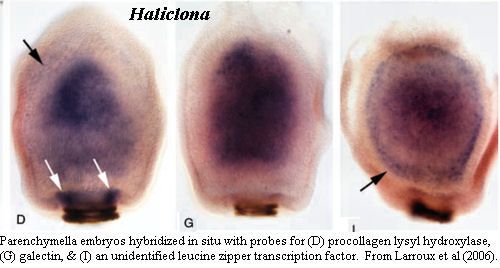
 Larroux
et al. (2006) is something of an exception. We have methodological (and
typological) issues with it, but it is another
Amazing Sponge Paper. After finding the requisite three layers and invoking
the G-word, they take a page or so of dithering to recover -- but they go on.
In particular, they go on with in situ hybridization experiments, the first in
any sponge. It is quite clear from these experiments that there are
more than three layers involved. There are either four or five layers.
At a minimum, distinct expression patterns are found in an additional "germ
layer" below (i.e. medial to) the subepithelial layer. In fact, this
layer can
even be faintly seen in the unstained embryo from the preceding figure.
The authors refer to this as the "inner part of the subepithelial cell mass,"
but we have to wonder whether they do so because they are expecting to see only three
layers. The cold hand of Ernst Haeckel still grips the world of sponges at
times.
Larroux
et al. (2006) is something of an exception. We have methodological (and
typological) issues with it, but it is another
Amazing Sponge Paper. After finding the requisite three layers and invoking
the G-word, they take a page or so of dithering to recover -- but they go on.
In particular, they go on with in situ hybridization experiments, the first in
any sponge. It is quite clear from these experiments that there are
more than three layers involved. There are either four or five layers.
At a minimum, distinct expression patterns are found in an additional "germ
layer" below (i.e. medial to) the subepithelial layer. In fact, this
layer can
even be faintly seen in the unstained embryo from the preceding figure.
The authors refer to this as the "inner part of the subepithelial cell mass,"
but we have to wonder whether they do so because they are expecting to see only three
layers. The cold hand of Ernst Haeckel still grips the world of sponges at
times.
The point we are trying to make is that sponges are not a bunch of sullen failures who are still sulking after flunking the Eumetazoan Entrance Examination 600 My ago. Sponges were, and are, the base of the entire metazoan radiation, and we ought to be unsurprised to discover such diversity of developmental pattern. Also (with a nervous glance around for the ghost of Ernst Haeckel) this adumbration of layers is most unlike the Calcarea. The calcarean sponges have two unique patterns. The calciblastula type involves essentially direct development from blastula to adult. The amphiblastula begins as a cup, with ciliated cells at the bottom, the cilia on the inside -- perhaps like an embryonic archaeocyath. The whole thing then flips inside-out, leaving the cilia facing outwards.. Ultimately, the non-ciliated macromeres overgrow everything. These modes do not bear any obvious relationship to Eumetazoa, or even Demospongiae.
What about the Homoscleromorpha? We thought you'd never ask. The homoscleromorph cinctoblastula is recognizably demosponge-like, but there are complications. In-and-out migration of individual cells, and cell migration along the outside, are both impossible due to the formation of the basement membrane. This has two results, one one of which favors a calcarean connection and one which does not. First, since epithelial micromeres cannot migrate laterally, metamorphosis into the adult form can only take place in the manner of some Calcarea. That is, the basal macromeres eventually overgrow the whole outer surface. Second, and unlike Calcarea, the micromeres can't migrate inward either, as happens in other sponge classes. Instead, multiplication of epithelial cells causes the outer layer to fold. Leys & Ereskovsky (2006). How much folding would it take to evolve an invaginating gut from one of those folds?
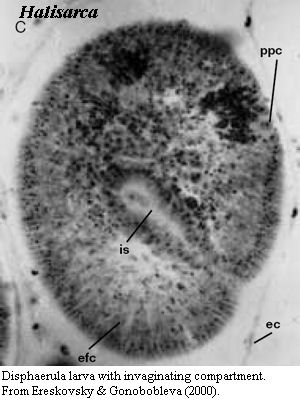
This is not a rhetorical question. Another group of demosponges, the Halisarcida, manage something quite like this, as the image from Ereskovsky & Gonobobleva (2000) shows. So is this gastrulation? It seems very unlikely. In fact, until we have a more sensible definition of gastrulation (which requires that we agree on what the homologies in gastrulation are), this is likely to remain a relatively meaningless question. What we may say, instead, is that the demosponge lineage seems to have a number of developmental characteristics shared with bilaterian gastrulation. These may, or may not, be synapomorphies; but, in any event, are characters which Demospongiae and Eumetazoa share with Homoscleromorpha, to the exclusion of Calcarea.
We may say all that -- but of course we may be completely wrong ...
| Page Back | Page Top | Unit Home | Page Next |