Bilateria-3
 In this final episode, we will attempt to do two things: (1) sum up the characteristics of Bilateria and (2) briefly review the candidate Middle Earthlings in view of those findings. Detailed comparison must be left for more detailed consideration of each particular kind of hobbit, orc, elf, etc.
In this final episode, we will attempt to do two things: (1) sum up the characteristics of Bilateria and (2) briefly review the candidate Middle Earthlings in view of those findings. Detailed comparison must be left for more detailed consideration of each particular kind of hobbit, orc, elf, etc.
The One Ring
One of the advantages of really profound ignorance is that it makes neutrality much easier. We began looking into this subject with very few preconceptions about Urbilateria. Having finished for now with this suppositional ancestor of all Bilateria, we find that he is a peculiar mixture of highly derived, and exceedingly primitive.
Urbilateria undoubtedly was a triploblast. That is, he had a fairly well-established system of mesoderm induction. Technau & Scholz 2003). Like cnidarians, he had muscles. Since he had a differentiated ectoderm separate from the gut, and also had a longitudinal layer of muscle and bilateral symmetry, he probably had some degree of motility. Indeed, he would almost have to have been mobile, unless he were fixed to the substrate. But a sessile lifestyle seems unlikely.Sessile organisms leave trace fossils in the form of holdfasts, variously reinforced holes in the substrate, and, frequently, mineral remains. Nothing like this is associated with bilaterians until the Small Shelly Fauna of the Terreneuvian. So, Urbilateria was either motile or he arrived extraordinarily late, in addition to being oddly designed for a benthic couch potato.
 There are several other reasons for believing Urbilateria was not sessile. One is trivial. Sessile aquatic animals are rarely very small. It is simply too easy for them to be buried in sediment. Yet we can be relatively confident that Urbilateria was indeed small, since there are no indications of anything large and bilaterian until the middle of the Early Cambrian. Of course, fossils from any earlier date are extremely rare, so some behemoth proto-planarian might yet show up. However, while the record of Neoproterozoic body fossils is poor, we have a large sample of worm-like trace fossils to examine, beginning in the Ediacaran. These are all quite small.
There are several other reasons for believing Urbilateria was not sessile. One is trivial. Sessile aquatic animals are rarely very small. It is simply too easy for them to be buried in sediment. Yet we can be relatively confident that Urbilateria was indeed small, since there are no indications of anything large and bilaterian until the middle of the Early Cambrian. Of course, fossils from any earlier date are extremely rare, so some behemoth proto-planarian might yet show up. However, while the record of Neoproterozoic body fossils is poor, we have a large sample of worm-like trace fossils to examine, beginning in the Ediacaran. These are all quite small.
The next proof is important: the complex gut. Arendt et al. (2001). Suspension feeding doesn't require a specialized mouth or stomadeum. In fact, that would be counterproductive. A suspension feeder wants a big surface area to catch potentially edible particles. Filter feeding is a possibility, but this requires rather specialized equipment, none of which seems to have survived as homologous structures in the main Bilaterian clades. The path of high probability is that Urbilateria moved -- if not much -- in search of food.
That leaves two possible lifestyles: on the substrate or in the water column. Kimberella seems to have been a pre-Cambrian bilaterian bottom-feeder. If so, at least some bilaterians had this niche. It has recently been shown that some Late Proterozoic bottoms may have experienced extensive, millimeter-scale horizontal bioturbation (Dornbos et al., 2005) -- presumably caused by bilaterians not yet able to penetrate the substrate. In any case, it is nearly impossible to imagine something this small and ungainly as a swimmer. Doubtless there were planktonic forms, but Urbilateria seems rather complex for life as a floater, and there would be little point in its various physical specializations. So, we conclude that Urbilateria was part of the benthic epifauna -- a bottom crawler.
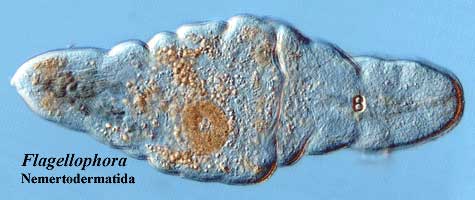
We have two sorts of physical models for Urbilateria. The trace fossils and Acoela suggest something long and generally worm-like. Kimberella and Xenoturbella look more like very primitive mollusks. Fortuitously, the living Nemertodermatida are, morphologically, more or less in the middle, which is probably our best bet. Finally, The evidence indicates that Urbilateria had a nervous system with one or (more likely) two main longitudinal nerve cords and an anteriorly-placed nerve plexus, sensory cells for the perception of light, and some kind of mechanosensory system.
 The Fellowship of the Ring
The Fellowship of the Ring
All this is excellent news, since it constitutes a reasonably good fit with some of the candidates for Middle Earth citizenship, particularly the Acoelomorpha. This is the name given to a supposed clade made up of Nemertodermatida and Acoela. Four other groups are possibilities. Two seem too primitive, and may well turn out to be weird Radiata: the Myxozoa and Mesozoa. Two others are probably too specialized and may turn out to be protostomes (and ecdysozoans in particular): the Chaetognatha and Gnathostomulida. Nevertheless, we will include them all in this section for now, although we'll probably change our minds before it's finished.
Acoela: The Acoela were classically shelved with the Platyhelminthes, those exceedingly dull worms which you quickly forgot after the first quiz in Introductory Zoology. You heard on good authority that the instructor never asks about them on the Final. This turned out to be true. In fact, it is always true. No instructor ever asks about the Platyhelminthes on the final. It is simply one of those unspoken laws of academic nature. So, how could we possibly prevail on you to stay awake long enough to absorb serious data on the Acoela -- a group of worms so cloddish and uncouth that they have been demoted from the platyhelminths to an even lower plane of phylospace? We will perform this impossible task by applying a time-honored academic maxim. "The difficult, we do immediately. The impossible, by pawning it off on someone else". See the excellent essay on this topic by Prof. Seth Tyler (U. 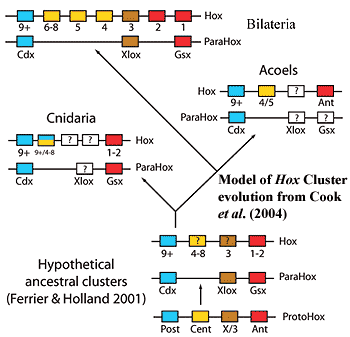 Maine), or pages 9-11 of Tekle (2006).
Maine), or pages 9-11 of Tekle (2006).
Tentatively, we disagree with almost all of Prof. Tyler's phylogenetic conclusions. The Acoela are quite specialized and weird, but this is only to be expected of a surviving twig at the base of an enormous radiation 600 My ago, or more. Further, the closely related Nemertodermatida provide a connecting link to more conventional bilaterian designs. The acoel gut is not a conventional 3-part bilaterian gut, but the longitudinal body wall muscle net, nerves, brain, mode of life, and body form are correct. For that matter, even the syncytial "gut" departs further from the cnidarian or poriferan system than from the bilaterian archenteron. Development proceeds in a recognizably bilaterian fashion, with a spiral cleavage pattern (albeit a spiral pattern different from that of annelids). Morphologically, the Acoela are simply well-behaved hobbits with full stomachs.
The one real hitch comes from Cook et al. 2004), who looked at acoel hox genes. To make a long and somewhat uncertain story shorter and more certain than it really is, at least one acoel has only three hox genes, rather than the usual bilaterian minimum of 7-8. Then again, as these authors note, nematodes also have a reduced hox set. In that case, it is generally thought that the number is due to gene loss. So, the number in Nemertodermatida may be due to a similar effect of small body size.
Nemertodermatida: The Nemertodermatida are a small group of acoels in rehab. They're trying to conform. They have guts. Sure, they've gained a little weight, but their nerves are better. Certainly, their sex lives are simpler and more conventional. Their developmental pattern is similar to that of acoels. Larsson 2003). They're taking it one day at a time.
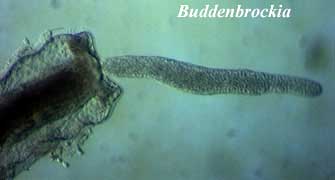 Myxozoa: The Myxozoa are odd parasites, originally thought to be protists (Brooke & Holland, 2003), with almost nothing to suggest that they are bilaterians -- usually. The Myxozoa are generally found as intracellular parasites with very complicated lives, including two hosts; for example, annelid and fish. Recently, Monteiro et al. (2002) realized that the mystery worm and putative bilaterian Buddenbrockia was actually an unrecognized form of the myxozoan, Tetracapsula. In this avatar, the myxozoan has body wall muscles in four regular muscle blocks and vaguely bilateral symmetry. Id.
Myxozoa: The Myxozoa are odd parasites, originally thought to be protists (Brooke & Holland, 2003), with almost nothing to suggest that they are bilaterians -- usually. The Myxozoa are generally found as intracellular parasites with very complicated lives, including two hosts; for example, annelid and fish. Recently, Monteiro et al. (2002) realized that the mystery worm and putative bilaterian Buddenbrockia was actually an unrecognized form of the myxozoan, Tetracapsula. In this avatar, the myxozoan has body wall muscles in four regular muscle blocks and vaguely bilateral symmetry. Id.
Initial molecular and morphological studies placed the myxozoans as close cousins of the parasitic cnidarian Polypodium. Later molecular work put them in the Bilateria. Halanych 2004). Nevertheless, outside the noisy clan of molecular enthusiasts, those who have actually studied Myxozoa seem to be convinced that myxozoans are, in fact, cnidarians. Some are polite enough not to say so directly. Kent et al. (2001). Others are less polite, possibly with good reason. Siddall & Whiting (1998). As Siddall & Whiting point out, it is hard to accept a bilaterian with nematocysts, and the polar capsules of Myxozoa are undoubtedly nematocysts modified as crowbars to break into host cells. Zrzavý 2001) (citing much other evidence).
 One obvious problem is, as we mentioned at the beginning, that few participants in these debates ever define their terms. It is not clear that any of these folks would assert that Myxozoa are bilaterians if they actually bothered to give Bilateria a phylogenetic definition. It is entirely possible that this entire dispute is, quite literally, about nothing. Nevertheless, we will treat the Myxozoa as presumptive Bilateria for now, simply for lack of a better place to deal with them.
One obvious problem is, as we mentioned at the beginning, that few participants in these debates ever define their terms. It is not clear that any of these folks would assert that Myxozoa are bilaterians if they actually bothered to give Bilateria a phylogenetic definition. It is entirely possible that this entire dispute is, quite literally, about nothing. Nevertheless, we will treat the Myxozoa as presumptive Bilateria for now, simply for lack of a better place to deal with them.
Mesozoa: The Mesozoa are another group of intracellular parasites -- unlikely candidates, you'd think, for bilaterian status. They are now believed to be made up of two, possibly unrelated, groups: the Dicyemida Rhombozoa) and Orthonectida. They are known to have hox-like genes, and one of the dicyemid genes has a lophotrochozoan signature. Some, generally conservative, workers have found that the Mesozoa are in fact monophyletic and that they branch near the Acoelomorpha. Siddall & Whiting (1998); Zrzavý (2001). This creates difficulties, since the Mesozoa have very little other than molecular traits to tie them to the Bilateria. However, so far as we are aware, the members of this taxon are all obligate intracellular parasites. So, extreme simplification is only to be expected.
Chaetognatha: As mentioned, the chaetognaths ("arrow worms") are clearly orthodox bilaterians. The question to be answered is, "why aren't they protostomes?" Classically, they were regarded as deuterostomes, but that now seems extremely unlikely. The chaetognaths were subsequently moved to the ecdysozoan region, but this became less likely based on subsequent detailed study of chaetognath development and neuroanatomy. The pattern of early development looks "spiralian," i.e. spiral cleavage with each of the early cells having a fixed role in the organism. (This is seems to be the primitive condition. Extavour & Akam, 2003.) A full complement of hox genes are present, but their expression is restricted to the central nervous system, as in certain other?) lophotrochozoans. Papillon et al. (2005). Thus, the current best bet is that the chaetognaths are either lophotrochozoans or basal to Protostomia. Chances are that they are lophotrochozoans, but mitochondrial gene order phylogenies place them in a more basal position.
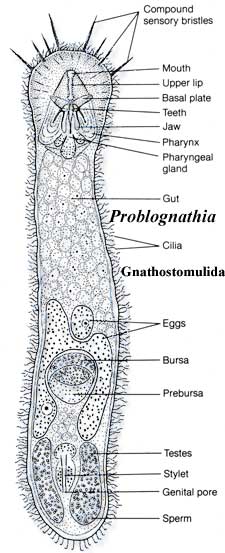
Gnathostomulida: This is, as you may have guessed, one more gutless group of worms. They differ from the others in having jaws of a sort. As far as we can make out, no one has seriously proposed that they are basal to Protostomia. Like the Acanthocephala, they are probably evil rotifers. However, no one seems particularly comfortable with them either. Consequently, we will add them here as candidate orcs. And, while we're at it, perhaps we'll do the Acanthocephala, too.
Perhaps we ought to be a bit more candid. Our reasons are actually not quite so frivolous -- almost as frivolous, but not quite. At least since the publication of an influential paper by Giribet et al. (2000), there has been a vague suspicion that the Ecdysozoa-Lophotrochozoa dichotomy masks a third, possibly rather large, clade. This clade -- admittedly of variable composition -- tends to show up in a polytomy with the other two. It might be basal to Protostomia bugs + slugs). It might not. Consequently, we have picked two likely members of the group, just to keep an eye on this possibility.
So. There we have it. Middle Earth, an imaginary land, has been populated with a hypothetical population of odd creatures. Just for fun, we now add our completely baseless and intuitive guess about how things will turn out in the next five to ten years.
Radiata = Cnidaria (i.e. Bilaterians are Cnidarians!)
├─Myxozoa
└─┬─Acoelomorpha
│ ├─Nemertodermatida
│ └─Acoela
└─Bilateria
├─Deuterostomia (by definition)
└─┬─Gnathostomulida
└─Protostomia (bugs + slugs)
├─Ecdysozoa (bugs > slugs)
│ ├─Acanthocephala?
│ └─bugs
└─Lophotrochozoa (slugs > bugs)
├─Chaetognatha
└─┬─Mesozoa
└─slugs
ATW060218
Arendt, D, U Technau & J Wittbrodt (2001), Evolution of the bilaterian larval foregut. Nature 409: 81-85.
Arendt, D & J Wittbrodt (2001), Reconstructing the eyes of Urbilateria. Phil. Trans. Roy. Soc. B 356: 1545-1563.
Brooke, NM & PWH Holland (2003), The evolution of multicellularity and early animal genomes. Curr. Op. Genet. Devel. 13: 599-603.
Cook, CE, E Jiménez, M Akam & Emili Saló 2004), The Hox gene complement of acoel flatworms, a basal bilaterian clade. Evol. Devel. 6: 154-163.
Dornbos, SQ, DJ Bottjer & J-Y Chen (2005), Paleoecology of benthic metazoans in the Early Cambrian Maotianshan Shale biota and the Middle Cambrian Burgess Shale biota: evidence for the Cambrian substrate revolution. Palaeogeog. Palaeoclimat. Palaeoecol. 220: 47– 67.
Erwin, DH & EH Davidson (2002), The last common bilaterian ancestor. Development 129: 3021-3032.
Extavour, CG & M Akam (2003), Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130: 5869-5884.
Ghysen, A (2003), The origin and evolution of the nervous system. Int. J. Dev. Biol. 47: 555-562.
Giribet, G, DL Distel, M Polz, W Sterrer & WC Wheeler (2000), Triploblastic relationships with emphasis on the acoelomates and the position of Gnathostomulida, Cycliophora, Plathelminthes, and Chaetognatha: A combined approach of 18S rDNA sequences and morphology. Syst. Biol. 49: 539-562.
Halanych, KM (2004), The new view of animal phylogeny. An Rev. Ecol. Evol. Syst. 35: 229-256.
Hanelt, B, D Van Schyndel, CM Adema, LA Lewis & ES Loker (1996), The phylogenetic position of Rhopalura ophiocomae (Orthonectida) based on 18S ribosomal DNA sequence analysis. Mol. Biol. Evol. 13: 1187–1191.
Hejnol, A & R Schnabel (2004), The eutardigrade Thulinia stephaniae has an indeterminate development and the potential to regulate early blastomere ablations. Development
132: 1349-1361.
Herlyn, H, O Piskurek, J Schmitz, U Ehlers, & H Zischler (2003), The syndermatan phylogeny and the evolution of acanthocephalan endoparasitism as inferred from 18S rDNA sequences. Mol. Phylogen. & Evol. 26: 155-164.
Hooge, MD & N Eppinger (2005), New species of Acoela (Acoelomorpha) from the Gulf of California. Zootaxa
1009, 14pp.
Kent, ML, KB Andree, JL Bartholomew, M El-Matbouli, SS Desser, RH Devlin, SW Feist, RP Hedrick, RW Hoffmann, J Khattra, SL Hallett, RJG Lester, M Longshaw, O Palenzeula, ME Siddalli & C-X Xiao 2001), Recent advances in our knowledge of the Myxozoa. J. Eukaryot. Microbiol. 48: 395-413.
Larsson, K (2003), Early Development of Nemertoderma westbladi. Unpub. Degree Project, Uppsala Univ., 13 pp. WWW.
Lartillot, N, M Le Gouar & A Adoutte (2002), Expression patterns of fork head and goosecoid homologues in the mollusc Patella vulgata supports the ancestry of the anterior mesendoderm across Bilateria. Dev. Genes Evol. 212: 551-561.
Lavrov, DV & BF Lang (2005), Poriferan mtDNA and animal phylogeny based on mitochondrial gene arrangements. Syst. Biol. 54: 651-659.
Levinton, J, L Dubb & GA Wray (2004), Simulations of evolutionary radiations and their application to understanding the probability of a Cambrian Explosion. J. Paleontol. 78: 31-38.
Meinhardt, H (2002), The radial-symmetric hydra and the evolution of the bilateral body plan: an old body became a young brain. BioEssays 24: 185-191.
Monteiro, AS, B Okamura & PWH Holland (2002), Orphan worm finds a home: Buddenbrockia is a myxozoan. Mol. Biol. Evol. 19: 968–971.
Papillon, D, Y Perez, L Fasano, Y Le Parco & X Caubit (2005), Restricted expression of a median Hox gene in the central nervous system of chaetognaths. Dev. Genes Evol. 215: 369–373.
Peterson, KJ, B Waggoner & JW Haggadorn 2003), A fungal analog for Newfoundland Ediacaran fossils? Integr. Comp. Biol. 43: 127-136.
Philippe, H, N Lartillot & H Brinkmann (2005), Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol. Biol. Evol. 22: 1246-1253.
Rieger, RM & P Ladurner (2003), The significance of muscle cells for the origin of mesoderm in Bilateria. Integr. Comp. Biol. 43: 47-54.
Rokas, A, N King, J Finnerty & SB Carroll (2003), Conflicting phylogenetic signals at the base of the metazoan tree. Evol. Devel. 5: 345-359.
Rokas, A, D Krüger & SB Carroll (2005), Animal evolution and the molecular signature of radiations compressed in time. Science 310: 1933-1938.
Ruiz-Trillo, I, J Paps, M Loukota, C Ribera, U Jondelius, J. Baguñà, & M Riutort (2002), A phylogenetic analysis of myosin heavy chain type II sequences corroborates that Acoela and Nemertodermatida are basal bilaterians. Proc. Nat. Acad. Sci. (USA) 99: 11246-11251.
Seaver, EC (2003), Segmentation: mono- or polyphyletic? Int. J. Dev. Biol. 47: 583-595.
Siddall, ME & MF Whiting (1998), Long-branch abstractions. Cladistics 15:9-24.
Steinauer, ML, BB Nickol, R Broughton & G Ortí (2005), First sequenced mitochondrial genome from the Phylum Acanthocephala (Leptorhynchoides thecatus) and its phylogenetic position within Metazoa. J. Mol. Evol. 60: 706-715.
Technau, U & CB Scholz (2003), Origin and evolution of endoderm and mesoderm. Int. J. Dev. Biol. 47: 531-539.
Tekle, YI (2006), Phylogeny and Taxonomy of Childia Acoela). Unpub. doctoral thesis., Uppsala Univ. WWW.
Wray, GA, JS Levinton & LH Shapiro (1996), Molecular evidence for deep precambrian divergences among metazoan phyla. Science 274: 568-573.
Zrzavý, J (2001), The interrelationships of metazoan parasites: a review of phylum- and higher-level hypotheses from recent morphological and molecular phylogenetic analyses. Folia Parasitol. 48: 81-103.
page uploaded MAK020407, last modified ATW050724
checked ATW050724
this material may be freely used as long as attribution is given
 In this final episode, we will attempt to do two things: (1) sum up the characteristics of Bilateria and (2) briefly review the candidate Middle Earthlings in view of those findings. Detailed comparison must be left for more detailed consideration of each particular kind of hobbit, orc, elf, etc.
In this final episode, we will attempt to do two things: (1) sum up the characteristics of Bilateria and (2) briefly review the candidate Middle Earthlings in view of those findings. Detailed comparison must be left for more detailed consideration of each particular kind of hobbit, orc, elf, etc.
 The Fellowship of the Ring
The Fellowship of the Ring Maine), or pages 9-11 of
Maine), or pages 9-11 of 
 One obvious problem is, as we mentioned at the beginning, that few participants in these debates ever define their terms. It is not clear that any of these folks would assert that Myxozoa are bilaterians if they actually bothered to give Bilateria a phylogenetic definition. It is entirely possible that this entire dispute is, quite literally, about nothing. Nevertheless, we will treat the Myxozoa as presumptive Bilateria for now, simply for lack of a better place to deal with them.
One obvious problem is, as we mentioned at the beginning, that few participants in these debates ever define their terms. It is not clear that any of these folks would assert that Myxozoa are bilaterians if they actually bothered to give Bilateria a phylogenetic definition. It is entirely possible that this entire dispute is, quite literally, about nothing. Nevertheless, we will treat the Myxozoa as presumptive Bilateria for now, simply for lack of a better place to deal with them.