 The Hypermineralized Cap
The Hypermineralized Cap| Bones: Teeth | ||
| The Vertebrates | Overview-2 |
| s | ||||||
| Vertebrates Home | Vertebrate | Vertebrate | Bones | Time |
| Bones
|
Teeth
|
 The Hypermineralized Cap
The Hypermineralized CapThe hypermineralized layer in mature individuals typically contains only 2% to 10% protein. When the protein content of bone drops to about 3% (i.e., 97% hydroxyapatite), the material is referred to as enamel or enameloid. The difference between enamel and enameloid is discussed in the next section. For the nonce, we focus on enamel. Sarcopterygians and closely related fishes have "true" enamel. Enamel is characterized developmentally by a protein template largely composed of amelogenin, a protein secreted by cells of the epithelium. Amelogenin binds tightly to calcium and -- oddly -- also to cell surfaces [H+02]. Initially, the cap area is almost all protein, 90% of which is amelogenin [MP00]. As the tooth matures, the protein matrix is gradually degraded until the fully developed hypermineralized condition is reached, with close packing of the enamel structures [D+01].
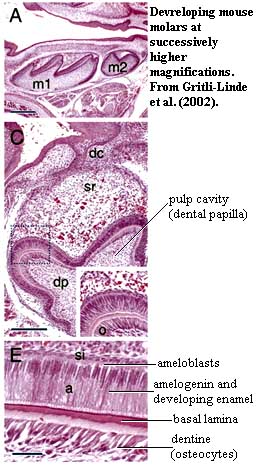 In most vertebrates, these enamel structures are formed of "semiparallel" crystallites of HAP which radiate outward from a rather distinct enamel-dentine junction [MP00]. This junction is clearly visible as a dark band or basal lamina in electron micrographs of developing vertebrate teeth. Most of these features can be seen in the image of the developing dental enamel in the frog, Rana. Mammals have evolved yet a further refinement on this system. Mammal teeth develop cylindrical or hemicylindrical enamel prisms. These prisms begin with "bundled" parallel crystallites, a structure found in many vertebrates, including sharks (see images at Synechodontiformes) and frogs (triple arrows in image at right). However, in mammals, these are bounded by other crystallites oriented at a sharp angle to the parallel bundles [MP00] (see image and text at glossary entry).
In most vertebrates, these enamel structures are formed of "semiparallel" crystallites of HAP which radiate outward from a rather distinct enamel-dentine junction [MP00]. This junction is clearly visible as a dark band or basal lamina in electron micrographs of developing vertebrate teeth. Most of these features can be seen in the image of the developing dental enamel in the frog, Rana. Mammals have evolved yet a further refinement on this system. Mammal teeth develop cylindrical or hemicylindrical enamel prisms. These prisms begin with "bundled" parallel crystallites, a structure found in many vertebrates, including sharks (see images at Synechodontiformes) and frogs (triple arrows in image at right). However, in mammals, these are bounded by other crystallites oriented at a sharp angle to the parallel bundles [MP00] (see image and text at glossary entry).
In all cases, the process of enamel growth and orientation is under the control of the specialized epithelial cells known as ameloblasts. Ameloblasts control the process less through the differential deposition of enamel than through the amelogenin matrix they construct. Mammals, again, add a further element of ameloblast control. Each mammal ameloblast has a terminal Tomes process which assists in creating and orienting the enamel prism created by that cell. The arrangement gets progressively more complicated in higher mammals, with several different amelogenins and the addition of other proteins to shape various different patterns of enamel deposition depending on the tooth type, enamel layer and location on the tooth [MP00].
Thus, the evolution of enamel largely relates to the evolution of amelogenin. Delgado et al [D+01] have tried valiantly to solve this riddle, but it turns out to be a particularly hard nut to crack. There are any number of problems. First, the amelogenin gene transcript is spliced in several different ways to yield several different mRNAs in the same organism [MP00]. Thus, part of the evolutionary story is hidden -- we can probably reconstruct the gene, but we can't tell how the alternate splicing forms developed, or at what phylogenetic level. Second, amelogenin is only used to develop enamel and the gene is lost quickly in all lines that don't use enamel. For example, turtles and birds, which have no teeth, have no amelogenin gene. Worse, the gene has absolutely no identifiable homologues. This is almost unprecedented. Despite these problems, Delgado & Co. develop an elaborate molecular phylogeny of amelogenin. However, their model is all based on the homologues of a single, very small, exon (transcribed gene segment) which codes for a peptide short sequence of amino acids) involved in protein transport through the endoplasmic reticulum. This purpose of this peptide is to allow amelogenin to be transported out of the cell. There is no indication that this peptide is one of the functional components of amelogenin, once exported, and there is no reason to believe that it can serve as a phylogenetic proxy for the rest of the protein.
We have taken enamel first, because we at least understand what enamel is, and its development is comparatively well understood in mammals and (albeit to a lesser extent) other tetrapods. Enameloid development, by contrast, is poorly understood. In fact, in order to discuss the topic at all, we are forced to impose our own, possibly heterodox, notions on the unfortunate and much-abused reader. Worse, we must do so without much in the way of literature support.
 Our tragic quest began in happy innocence, with a routine search for a pithy, consensus definition of "enameloid" with which to enliven this turgid discourse. Our naive curiosity soon turned to bewilderment, rapidly degenerating into a desperate and disorganized hunt for any kind of definition at all. A few hours later, out of the depths of a swirling maelstrom of paleodental chaos, it suddenly became very clear -- from no particular source -- that the absence of a positive definition enameloid is an inevitable consequence of its true nature. Enameloid is not a particular structure, but simply the name we give to a continuum of hard tissues. Enamel lies at one extreme of this continuum. A theoretical dentine-like substance lies at the other extreme.
Our tragic quest began in happy innocence, with a routine search for a pithy, consensus definition of "enameloid" with which to enliven this turgid discourse. Our naive curiosity soon turned to bewilderment, rapidly degenerating into a desperate and disorganized hunt for any kind of definition at all. A few hours later, out of the depths of a swirling maelstrom of paleodental chaos, it suddenly became very clear -- from no particular source -- that the absence of a positive definition enameloid is an inevitable consequence of its true nature. Enameloid is not a particular structure, but simply the name we give to a continuum of hard tissues. Enamel lies at one extreme of this continuum. A theoretical dentine-like substance lies at the other extreme.
What all enameloids seem to have in common is a significant mesenchymal component to the protein matrix in which the mineral (not always hydroxyapatite) is laid down. That is, rather than amelogenin dropping like gentle rain from epithelial ameloblasts outside the basal lamina, other proteins, rising like writhing worms from odontoblasts in the depths of the underlying mesenchyme, form some or all of the protein matrix. The protein in question is usually, or predominantly, collagen, but other proteins may play important roles.
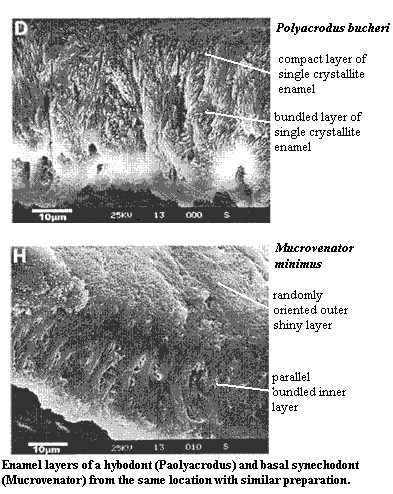
In real life, there is no clean differentiation. Stuff that looks suspiciously like true enamel still appears adjacent to, or as a layer of, many enameloids. Basal actinopterygians sometimes have both substances. Living sharks have no true enamel (we are told), but amelogenin mRNA transcripts can be found around the developing tooth. In fact, the enameloid teeth of neoselachians, urodeles and some teleosts has three layers: (a) a rather dense layer of rather randomly-oriented small crystallites, often with considerable residual collagen, near the basal lamina, (b) a layer of long parallel crystallites, which may or may not be bundled into distinct strands, and an amorphous surface layer [C+01] [D+02]. Unfortunately, there appears to be little phylogenetic importance to this arrangement, since more primitive elasmobranchs have a completely different arrangement involving an inner parallel bundled layer and a more enamel-like outer, single-crystallite layer [C+01]. Hence the lack of any dependable definitions for enameloid. The substance varies even within teeth and between organisms, depending on the nature and degree of odontoblast (or perhaps other mesenchymal) participation.
Update: We have now been enlightened by Gillis & Donoghue (2007). To our surprise and dismay, it turns out that this description is essentially correct. Frankly, we were hoping the story would turn out to be more strange and interesting -- but we will settle for comprehensibility. The authors define enameloid as "a hypermineralized tissue with a matrix of mixed ameloblastic (ectodermal) and odontoblastic (ectomesenchymal) origin." SEMs of living and fossil material shows that enameloid is probably plesiomorphic for gnathostomes. ATW080227 |
Whatever the protein matrix, mineralization proceeds more or less as in enamel teeth [D+02]. That is, mineralization begins with the deposition of tiny amorphous mineral grains. These serve as nuclei for the formation of small, randomly oriented crystal platelets 5-20 nm in diameter [D+02]. These platelets may not be apatite, but rather calcium carbonate or phosphate in the process of conversion to apatite. In any event, the small platelets probably coalesce to form the long crystals which soon dominate the enameloid layer. Presumably, in enameloid layers with parallel crystallites, the protein matrix is so arranged that an elongating crystal is increasingly forced to orient along an axis perpendicular to the basal lamina [D+02].
The further morphogenesis of the cap region is also rather variable. For example, in mouse teeth, Shh transcription ends fairly soon after the organizer is formed. Secondary organizers take over cusp development in mammals, but it is uncertain what, if any role shh plays in this process. By contrast, in elasmoid scales, Shh appears quite late and continues to be produced in a posteriorly migrating zone equivalent to the basal lamina of teeth and (presumably) ancient scales [SA04]. However, both of these are highly derived systems, and it would probably be a mistake to assume that either is typical of development in deep time.
 Now, if all this is true -- though we make no promises and disclaim all warranties -- we may be able to trace some of the main themes in the evolution of the system.
Now, if all this is true -- though we make no promises and disclaim all warranties -- we may be able to trace some of the main themes in the evolution of the system.
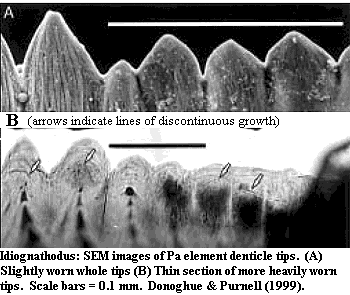
Perhaps the most basal system in which denticulate structures are reasonably well known is the feeding apparatus of conodonts. The general form and structure of these odd "teeth" is discussed in more detail elsewhere. For our purposes here, we focus on the structure of the denticles, as shown in the image from Donoghue & Purnell [DP99]. In the image, note the fibrous structure of the denticle tips which extends almost to the end of the denticle. In cross-section, the structure of the tips is dense, but with a rather woven texture. This suggests an enameloid based on a collagen matrix, as Sansom et al. [S+92] have concluded. As Donoghue & Purnell point out, growth was clearly discontinuous. To judge from the literature, each growth cycle adding a layer of 1-5µ mineralized tissue. From the cross section, we can see that the pulp cavity was retreating, becoming larger, but also more distant from the tip, with each growth cycle. Thus, it seems unlikely that collagen was produced from pulp odontoblasts as in teeth. It must have been applied, probably parallel to the surface, by an epithelial layer which periodically covered the denticles, as Donoghue & Purnell suggest [7].
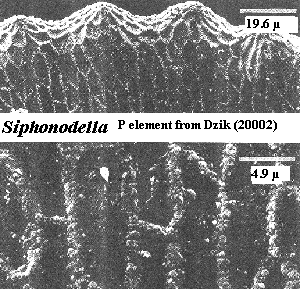 However, it is harder to accept the conclusion of some conodont workers that this is a gnathostome-style dentine-plus-enamel system [DS02]. Dzik [D00], for example, refers to the secretory cells as "ameloblasts" although his work shows quite clearly that these cells behaved as an epithelial monolayer which primarily deposited mineralized tissue as amorphous clumps in the spaces between adjacent cells.
However, it is harder to accept the conclusion of some conodont workers that this is a gnathostome-style dentine-plus-enamel system [DS02]. Dzik [D00], for example, refers to the secretory cells as "ameloblasts" although his work shows quite clearly that these cells behaved as an epithelial monolayer which primarily deposited mineralized tissue as amorphous clumps in the spaces between adjacent cells.
In short, the gross morphology of conodont denticles generally resembles an odontode, but the fine structure is clearly laminar, rather fibrous, and without elongate crystals. Our interpretation of the histology is that we are seeing something close to enameloid, but applied as a series of very thin layers and without extensive crystallite development.
Continued on Next Page
checked ATW030403