Hox Genes - 4
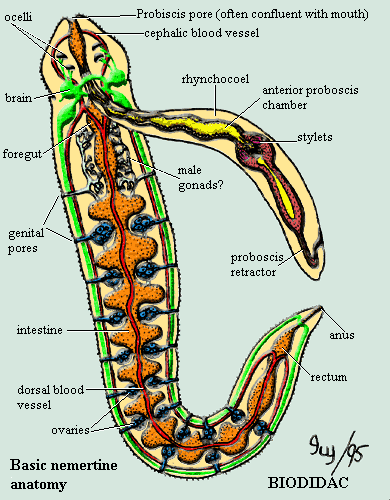 As we've mentioned, the phylogeny of Bilateria is undergoing a sort of counter-reformation, so it is appropriate that we begin with a Diet of Worms. Actually, we have always entertained unorthodox ideas about the nemertine (= nemertean) worms. You will be thinking that no one is to be trusted who spends any amount of time pondering the nemertines. Perhaps you are correct. In fact, we gave up nemertines for a number of years when the winds of phylogenetics blew them away from the key position we thought they might occupy. Now, Nemertini = Nemertea, Nemertinea, or even Rhynchocoela) may return to a place very near to Urbilateria.
As we've mentioned, the phylogeny of Bilateria is undergoing a sort of counter-reformation, so it is appropriate that we begin with a Diet of Worms. Actually, we have always entertained unorthodox ideas about the nemertine (= nemertean) worms. You will be thinking that no one is to be trusted who spends any amount of time pondering the nemertines. Perhaps you are correct. In fact, we gave up nemertines for a number of years when the winds of phylogenetics blew them away from the key position we thought they might occupy. Now, Nemertini = Nemertea, Nemertinea, or even Rhynchocoela) may return to a place very near to Urbilateria.
Nemertines are long, sometimes absurdly long (30m!), non-segmented worms. They are often brightly colored and very thin -- hence the name "ribbon worm" = "ribbonworm"). They are armed with something resembling a single enormous nematocyst, essentially a harpoon gun. Anatomically, they're rather basic except for the rhynchocoel. For much more (and better) information, see the Nemertes site.
Kmita-Cunisse et al. (1998) found that the nemertine Lineus had (probably) a single hox cluster with six (or possibly seven) hox genes. Oddly, the Lineus hox genes fell into homology classes 1, 3, 4, 6, 7, and 9. The absence of a hox2 homologue is peculiar, since we know that hox2 was one of the first homology groups to evolve. In addition, Kmita-Cunisse et al. note that almost all of the hox protein sequences are closer to Branchiostoma and vertebrates than to Drosophila. However, these are raw "percentage identical amino acid" scores. They are not particularly meaningful where, as here, the difference is generally 1-2 residues.
The Chaetognatha are another heretical phylum. Chaetognaths ("arrow worms") are constructed a bit like chordates who have somehow been rotated through varying angles. They have a caudal fin, but it is horizontal, rather than vertical. They have something resembling a notochord, but it is ventral. Historically, they were regarded as aberrant deuterostomes, but have recently moved in with the bugs and slugs.
Like Lineus, the chaetognath Spadella has a single, well-organized hox cluster with seven hox genes. These again include only one anterior gene, one hox3, four middle class genes, and a "mosaic gene that shares features of both median and posterior classes." Papillon et al. 2005).
To sum up, the two best-known lophotrochozoan hox systems seem to share the following features:
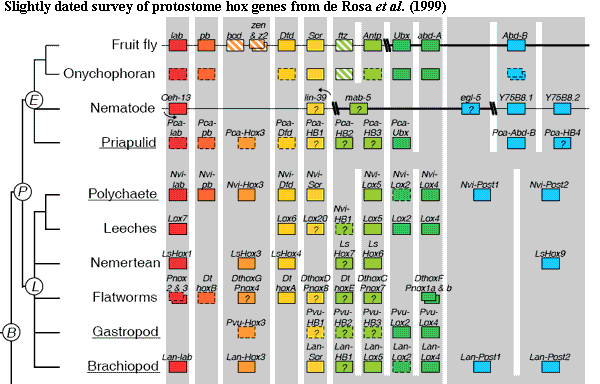 (a) absence of a hox2 homologue,
(a) absence of a hox2 homologue,
b) middle class hox genes are numerically dominant,
c) a maximum of 2 posterior hox, and
d) a single hox cluster (maybe).
The pattern holds, in a smudgy kind of way, over the entire clade. Unfortunately, the exceptions are significant. As you have probably realized, precious few generalizations in hoxworld can survive contact with phylogenetics. The hox data is wrong, the phylogenies are wrong, or hox genes are oddly homoplastic -- probably a bit of all three.
In particular, hox2 homologues have been reported from the gastropod Haliotis, and several polychaete worms (e.g., Chaetopterus). On the other hand, no hox2 homologue is known from cephalopod molluscs, the basal eogastropod limpet) Patella, non-polychaete annelids (e.g., the leech Helobdella), or the brachiopod Lingula. Lee et al. (2003); Callaerts et al. (2002); Irvine & Martindale (2001); Peterson et al. (2000); Shankland & Seaver (2000) de Rosa et al. (1999). Where it does show up, the lophotrochozoan hox2 tends to be peculiar. For example, in several cases, only part of the gene was found. Callaerts et al. suggested the presence of an unusual intron, although this should only be an issue for methods which rely on expressed RNAs. In other cases, the pattern of expression is anomalous. The hox2 homologue is expressed at an unexpected point in development and in a large area not easily related to any anatomical pattern of colinearity.
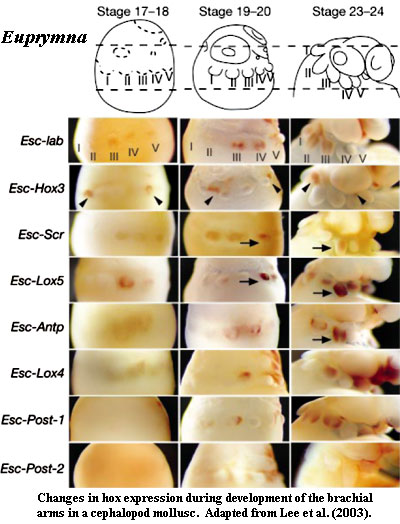 The remaining characteristics are more consistent, but less distinctive. Callaerts et al. (2002) believe that the ancestral mollusk had five central class genes, i.e. the full complement for Bilateria. However, it is far from clear that these are the same five genes which were inherited by deuterostomes, or even by arthropods. We understand that more recent work may actually increase the number of basal molluscan genes, but we have no details.
The remaining characteristics are more consistent, but less distinctive. Callaerts et al. (2002) believe that the ancestral mollusk had five central class genes, i.e. the full complement for Bilateria. However, it is far from clear that these are the same five genes which were inherited by deuterostomes, or even by arthropods. We understand that more recent work may actually increase the number of basal molluscan genes, but we have no details.
The real problem with molluscan hox systems is that molluscs have completely lost interest in traditional anteroposterior colinearity. This is not very surprising, since many molluscs practice a sort of developmental yoga which inspires them to contort the body axis in ways otherwise known only to devotees of the Kama Sutra. Perhaps for this reason, molluscs tend to use hox systems for spatial patterning in very precise ways, often involving multiple programs, as in Ciona. However, unlike the urochordate case, none of the molluscan programs could reasonably be described as colinear, or even anteroposterior. In fact, some of the programs seem to involve hox combinations which change over time, as well as space.
As with the more ambitious asana of the Kama Sutra, it isn't obvious how molluscs got into this position. Pappillon et al. 2005) make the interesting observation that mesodermal hox expression, particularly central hox expression is related to segmentation. The correlation between mesodermal hox expression and segmentation does seem to hold for lophotrochozoans. In fact, it holds reasonably well everywhere except in the sea urchin, in which all organized hox expression is mesodermal, despite the lack of segmentation. Arenas-Mena et al. 2000). Saying anything this definite about hox systems causes us no end of angst. We can only state that we spent the better part of a day checking the last couple of sentences, and could find no other exception. In particular, we were unable to identify any taxon with metameric segmentation but without mesodermal hox expression. At the same time, none of the various known molecular systems for segmentation involve hox genes, mesodermal or otherwise. Seaver (2003).
Lophotrochozoans are very poorly known, particularly considering how common, diverse, and significant they are. The evo-devo literature about them therefore tends to resemble the state of vertebrate evo-devo, circa 1990, including a great many fascinating, isolated factoids connected only by gossamer filaments of speculation. We leave the subject with one of these random gleanings. Like vertebrates, the cephalopods have evolved large eyes and substantial forebrains. In vertebrates, these critical novelties are developed in a hox-free anterior zone. Notwithstanding the very different workings of cephalopod hox systems, the eyes and forebrain (i.e., the cerebral ganglion) are likewise developed in a special, hox-free anterior zone. Lee et al. (2003).
 We're going to rip through the rest of the animals like a kangaroo on a pogo stick. We take this shortcut for a variety of discordant reasons. First, the arthropod (and related) systems have been intensively studied and the relationship of arthropod hox systems to the arthropod body plan is very well known. Carroll (2005) explains the whole thing in detail. Any attempt to redigest his very accessible writing on this topic would be pointless -- and, more to the point, boring for us. Second, we took on the hox project because we found we couldn't write a section on Crustacea without a clearer understanding of hox systems. Our crustacean section will therefore cover that region of arthropod phylospace, including more basal arthropods.
We're going to rip through the rest of the animals like a kangaroo on a pogo stick. We take this shortcut for a variety of discordant reasons. First, the arthropod (and related) systems have been intensively studied and the relationship of arthropod hox systems to the arthropod body plan is very well known. Carroll (2005) explains the whole thing in detail. Any attempt to redigest his very accessible writing on this topic would be pointless -- and, more to the point, boring for us. Second, we took on the hox project because we found we couldn't write a section on Crustacea without a clearer understanding of hox systems. Our crustacean section will therefore cover that region of arthropod phylospace, including more basal arthropods.
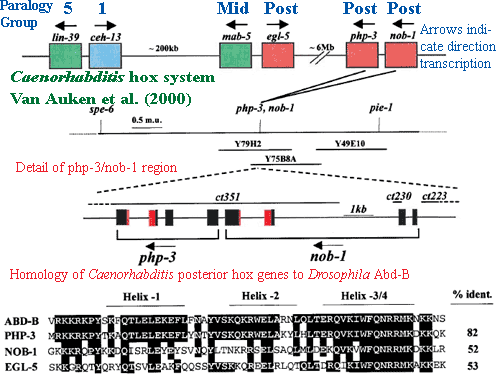
That leaves us with the base of the arthropod clade and the non-arthropod Ecdysozoans. This region consists largely of nematodes and is scientifically pathological. Much of the literature in that area is based on (a) phylogenetic assumptions that are contentious (Peterson et al., 2005) or (b) on Caenorhabditis, which turns out to be somewhat aberrant, even for a nematode (see, e.g., Valentine et al., 1999).
The hox set of Caenorhabditis is reduced and scrambled. Cook et al. (2004). Caenorhabditis has six hox genes, plus the usual three parahox genes. Saló et al. (2001). They are all found on one chromosome, but in three, widely-spaced pairs. Van Auken et al. 2000). In fact, none of the hox genes are particularly closely spaced except the php-3 + nob-1 pair which are separated by only about 200 base pairs. Id.
The homology of the nematode genes is weak in comparison to most of the others we have looked at. The hox1 homologue (ceh-13) is the closest to the general run of hox genes. Lin-39 has been somewhat ambiguously described as "an orthologue of pb, Dfd and Scr." Brunschwig et al. (1999). That is to say, it isn't even certain whether  the gene is an anterior or middle class hox. Of the posterior genes, php-3 seems to reasonably close to the typical Abd-B gene of Drosophila and other ecdysozoans. The other two posterior genes are not closely related to anything, including the two characteristic posterior genes of lophotrochozoans. de Rosa et al. (1999).
the gene is an anterior or middle class hox. Of the posterior genes, php-3 seems to reasonably close to the typical Abd-B gene of Drosophila and other ecdysozoans. The other two posterior genes are not closely related to anything, including the two characteristic posterior genes of lophotrochozoans. de Rosa et al. (1999).
A number of workers have argued that Caenorhabditis hox expression is, at least in part, anatomically colinear. Brunschwig et al. (1999); Wittman et al. (1997); Van Auken et al. (2000). While there is certainly something to this, we recommend a trip to WormBase, and a brief perusal of the expression patterns for any of the Caenorhabditis hox genes. The patterns are complex -- specific, but not easily characterized as "anterior" or "posterior," except in a rather general sense. Then again, perhaps this is actually the case with hox expression in most animals and is only obvious in Caenorhabditis because it is the most completely characterized animal developmental system.
At least some of the Caenorhabditis hox genes seem to operate by controlling cell motility and cell-cell interactions, rather than cell fates. Eisenmann et al. 1998) (lin-39) (but c.f. Yang et al., 2005); Brunschwig et al. (1999) (ceh-13); Pearson et al. 2003) (lin-39 and mab-5). We thought that this sounded like an interesting possibility for the basal function of hox genes. Unfortunately, it turns out that Caenorhabditis hox are not only unusual, but relatively unimportant. In fact loss of function mutations are lethal only for ceh-13 (hox1) and a small proportion of php-3 and nob-1 mutants. Van Auken et al. (2000). As these authors point out, the posterior hox mutants are hypomorphs. That is, the posterior hox genes seem to act in a quantitative way, so that loss of function only downregulates the expression of other genes. The hox genes are not acting as on/off switches, as they often do in more conventional hex systems.
Obviously, we've left off the critical arthropods. We'll get to them, and a conclusion, after we take time out to do the crustaceans.
page ATW070616
checked ATW070725
 As we've mentioned, the phylogeny of Bilateria is undergoing a sort of counter-reformation, so it is appropriate that we begin with a Diet of Worms. Actually, we have always entertained unorthodox ideas about the nemertine (= nemertean) worms. You will be thinking that no one is to be trusted who spends any amount of time pondering the nemertines. Perhaps you are correct. In fact, we gave up nemertines for a number of years when the winds of phylogenetics blew them away from the key position we thought they might occupy. Now, Nemertini = Nemertea, Nemertinea, or even Rhynchocoela) may return to a place very near to Urbilateria.
As we've mentioned, the phylogeny of Bilateria is undergoing a sort of counter-reformation, so it is appropriate that we begin with a Diet of Worms. Actually, we have always entertained unorthodox ideas about the nemertine (= nemertean) worms. You will be thinking that no one is to be trusted who spends any amount of time pondering the nemertines. Perhaps you are correct. In fact, we gave up nemertines for a number of years when the winds of phylogenetics blew them away from the key position we thought they might occupy. Now, Nemertini = Nemertea, Nemertinea, or even Rhynchocoela) may return to a place very near to Urbilateria. (a) absence of a hox2 homologue,
(a) absence of a hox2 homologue, The remaining characteristics are more consistent, but less distinctive. Callaerts et al. (2002) believe that the ancestral mollusk had five central class genes, i.e. the full complement for Bilateria. However, it is far from clear that these are the same five genes which were inherited by deuterostomes, or even by arthropods. We understand that more recent work may actually increase the number of basal molluscan genes, but we have no details.
The remaining characteristics are more consistent, but less distinctive. Callaerts et al. (2002) believe that the ancestral mollusk had five central class genes, i.e. the full complement for Bilateria. However, it is far from clear that these are the same five genes which were inherited by deuterostomes, or even by arthropods. We understand that more recent work may actually increase the number of basal molluscan genes, but we have no details.

