Hox Genes - 3
 When we get to Bilateria things are much clearer. We can triangulate back from Drosophila and vertebrates to get a reasonable idea what Urbilateria had in the way of a hox complement. Certainly it had both members of the anterior homology group, i.e. hox1 and hox2. It had at least one (and probably only one) posterior group genes. The Standard Model asserts that it had a hox3. That's a bit less clear, but still very likely. Finally, Urbilateria had a startling three central class genes. García-Fernàndez, 2005). Look back at the homology table. If those homologies are correct, this triangulation must be correct as well. Consequently, Urbilateria must have had a rather highly developed system of hox genes.
When we get to Bilateria things are much clearer. We can triangulate back from Drosophila and vertebrates to get a reasonable idea what Urbilateria had in the way of a hox complement. Certainly it had both members of the anterior homology group, i.e. hox1 and hox2. It had at least one (and probably only one) posterior group genes. The Standard Model asserts that it had a hox3. That's a bit less clear, but still very likely. Finally, Urbilateria had a startling three central class genes. García-Fernàndez, 2005). Look back at the homology table. If those homologies are correct, this triangulation must be correct as well. Consequently, Urbilateria must have had a rather highly developed system of hox genes.
Using the same technique, we can also deduce that Urbilateria's hox system was clearly identified with the antero-posterior axis, rather than a complex mixture of at least two axes, as in Nematostella. In fact, now that the confusing Acoelomorpha are removed from Bilateria, we can deduce that Urbilateria was a rather complex organism. Some of the morphological evidence and consequences are discussed at Bilateria (perhaps too cautiously, as matters have developed).
Some recent phylogenetic studies suggest why our last common ancestor with fruit-flies appears to be so remarkably advanced. See, e.g., Rogozin et al. 2007); Wang & Caetano-Anollés (2006); Wolf et al. (2004). These papers use powerful new statistical techniques and enormous datasets. The techniques are possibly too new and undeveloped to be really reliable -- but the basic approach used in all three papers is sound: they all look for events much rarer than single-nucleotide mutations. About time. These studies suggest that the nematodes may also have to be moved out of Bilateria. If so, we will probably have to resurrect the term Platyhelminthes at least to describe a large, paraphyletic grade of "flatworms" basal to the last common ancestor of flies and fish.
At that point, the whole concept of Bilateria becomes shaky and we may revert to the old "Coelomata" terminology, as argued in Wolf et al. (2004). We'll stick with the bilaterian terminology for another year or so, but it looks like another major shake-up of the animals is almost inevitable. In fact, the hox system alone almost compels the same result. We now understand Urbilateria's hox and parahox systems in some detail. But if it had a system of such complexity, can we accept that flatworms and nematodes descended from that ancestor?
Another aspect of the hox system which may be associated with the bilaterian body plan is a phenomenon we might call tagmatization; Pearson et al. (2005) put it like this: "In animal embryos in which mid-head and posterior abdomen can be  distinguished, ‘head’ Hox genes have their initial anterior boundaries of expression in epidermal, neural and mesodermal cells of the mid-head region, and ‘tail’ Hox genes have their initial anterior boundaries of expression in the corresponding cell types of the posterior abdomen." The only citation for this remark is a
1992 paper, and a great deal has happened in the 15 years since that paper was published. We would, for example, take issue with the "posterior abdomen" part.
distinguished, ‘head’ Hox genes have their initial anterior boundaries of expression in epidermal, neural and mesodermal cells of the mid-head region, and ‘tail’ Hox genes have their initial anterior boundaries of expression in the corresponding cell types of the posterior abdomen." The only citation for this remark is a
1992 paper, and a great deal has happened in the 15 years since that paper was published. We would, for example, take issue with the "posterior abdomen" part.
Nevertheless, it is broadly true that the anterior, posterior, and middle hox classes are often associated with major body divisions in Bilateria. And not just three divisions but, probably, five. Notice that Pearson et al. state that the anterior group begins in the "mid-head" region. All, or almost all animals with a head (and some without one) have a very significant pre-hox region. In addition, it has recently been proposed that protostomes also have a post-hox region behind the genital area. Copf et al. (2003); Schramm & Koenemann (2004). What about deuterostomes? Let's have a look.
The most distinctive hox character of the deuterostomes seems to be the multiplication of the posterior class. Most echinoderms have six genes in the posterior class. The same applies to some vertebrates, cephalochordates and some urochordates. Despite the tendency to converge on the number six, the gene phylogenies suggest that the deuterostome posterior hox group is not the result of some massive early multiplication event. Rather, the process was more gradual and often involved the duplication of different genes in different lineages. Monteiro & Ferrier (2006). However, it is also obvious from the weird morphology of the earliest deuterostomes (Yunnanozoa, Vetulicolia) that something significant happened to deuterostome posterior morphology quite early on, presumably associated with some expansion of the posterior hox class. Shu (2005) [8].
 The current belief is that the gut extended all the way through the "tail" region in Vetulicolia. Shu et al. (2001)*. The same is true of the contemporary Vetulocystidae, whose body plan is otherwise quite different. Shu et al. (2004)*. So, the deuterostome posterior extension is not merely an appendage, but a distinct body region (or tagma). However, it may very well be a hox-related region, since the posterior hox class seems to have expanded in all significant deuterostome groups.
The current belief is that the gut extended all the way through the "tail" region in Vetulicolia. Shu et al. (2001)*. The same is true of the contemporary Vetulocystidae, whose body plan is otherwise quite different. Shu et al. (2004)*. So, the deuterostome posterior extension is not merely an appendage, but a distinct body region (or tagma). However, it may very well be a hox-related region, since the posterior hox class seems to have expanded in all significant deuterostome groups.
The Echinoidea (sea urchins) appear to be unique in having completely rearranged the linear relationships of the hox system. In the urchin Strongylocentrotus, the anterior class hox genes and hox3 are found (a) downstream of the posterior class and (b) in reverse order. Richardson et al. 2005) [7]. As you might expect, the hox1, 2, and 3 homologues are oriented backward -- but so also are hox5 and hox11/13b. The urchin evx gene is still the downstream bookend to the system, but now located next to hox1, instead of the most posterior hox gene (here, hox11/13c).
But what does "posterior" mean to a radially symmetrical sea urchin? The answer is complex. Only two hox genes are expressed during urchin embryonic development, hox7 and hox11/13b. However, echinoid development is indirect. The sequence hox7, 8, 8/10, 11/13a, 11/13b is used, in an anatomically collinear way, and from mouth to anus, as the larva curls around the pentameric adult rudiment to form the radially symmetrical adult body. Arenas-Mena et al. 2000). What's more, all or almost all of the hox complement continues to be expressed in the adult.
 There seems to be a general tendency in the deuterostomes for hox1-3 to lie at some distance from hox5+, with hox4 variably placed nearer one group or the other.
See several vertebrate examples in Kim et al. (2000). Perhaps this is related to the tagmatization of the head region in deuterostomes -- the development of the head as a very different part of the body. Yet hox3 seems to be a sort of weak link in the hox chain for all bilaterians. It simply manifests itself in different ways. In the sea urchin, a break here seems to have promoted radial symmetry. In the vertebrates, the development of the head. In arthropods -- but we are getting a head of ourselves, so to speak ...
There seems to be a general tendency in the deuterostomes for hox1-3 to lie at some distance from hox5+, with hox4 variably placed nearer one group or the other.
See several vertebrate examples in Kim et al. (2000). Perhaps this is related to the tagmatization of the head region in deuterostomes -- the development of the head as a very different part of the body. Yet hox3 seems to be a sort of weak link in the hox chain for all bilaterians. It simply manifests itself in different ways. In the sea urchin, a break here seems to have promoted radial symmetry. In the vertebrates, the development of the head. In arthropods -- but we are getting a head of ourselves, so to speak ...
We leave the bizarre Echinoidea with one random observation. Remember Nematostella, the other radially symmetrical animal we've discussed? Recall that evx was also adjacent to hox1 (but upstream) in the sea anemone, like the sea urchin. It's probably just a coincidence -- but curious all the same.
Like the sea urchins, urochordates (tunicates) develop indirectly, through a morphologically distinct juvenile stage. The tunicate juvenile is motile and has a distinctively chordate body plan. The adult is sessile and the body is laid out more along the lines of a mollusk. The hox clusters of urochordates are often said to have "disintegrated." Varying numbers of middle and/or posterior class hox genes are missing, the various hox genes have gone their 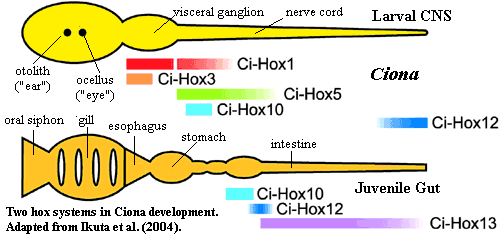 separate ways, and genetic colinearity tends to be lost. These trends are taken to extremes in the larvacean Oikopleura, which has only one remaining middle class hox and no contiguous hox genes. However the Larvacea tend to be extreme minimalists, often dispensing with whole organ systems heart, gills, etc.) and great swathes of DNA, in a profligate manner entirely unbecoming of a chordate. Swalla et al. (2000).
separate ways, and genetic colinearity tends to be lost. These trends are taken to extremes in the larvacean Oikopleura, which has only one remaining middle class hox and no contiguous hox genes. However the Larvacea tend to be extreme minimalists, often dispensing with whole organ systems heart, gills, etc.) and great swathes of DNA, in a profligate manner entirely unbecoming of a chordate. Swalla et al. (2000).
Ciona, an ascideacean, takes a more restrained approach. Its hox genes are split up among five clusters, and a number of central and posterior elements are lost. However, Ciona retains a peculiar kind of morphological colinearity. Ikuta et al. (2004). As the diagram indicates, the Ikuta group argues that two separate hox programs are being run off the same set of hox genes: one establishes the larval central nervous system, while the second patterns the juvenile gut. From the figures, we speculate that perhaps there is yet a third hox program which works in the larval head at metamorphosis (see additional figure from Ikuta et al.).
Again we find that genetic and anatomical colinearity are not tightly related. The genetic grouping (hox1, hox2-4, hox5-6, hox10, hox12-13) isn't correlated in an obvious way with any of the developmental programs.  On the other hand, perhaps Ciona suggests something about the echinoids. It is interesting that two groups of organisms, both of which go through extremely indirect development, both also have fractured hox clusters. As we'll see, indirect development and hox rearrangement are not Siamese twins, joined at the hip, but they are sufficiently correlated to be more than casual acquaintances. It may be a natural (but not inevitable) result of running multiple genetic programs off the same set of hox genes.
On the other hand, perhaps Ciona suggests something about the echinoids. It is interesting that two groups of organisms, both of which go through extremely indirect development, both also have fractured hox clusters. As we'll see, indirect development and hox rearrangement are not Siamese twins, joined at the hip, but they are sufficiently correlated to be more than casual acquaintances. It may be a natural (but not inevitable) result of running multiple genetic programs off the same set of hox genes.
By contrast to urochordates, the cephalochordate Branchiostoma (= amphioxus) has the archetype of all hox systems: a single, unbroken cluster of
14 hox genes, all neatly arranged in order.
 We're going to have to weasel out here. We will not attempt to cover the vertebrates in detail. This is the Invertebrate section of Palaeos, after all. That is a reasonable excuse -- and almost credible. Can we help it if vertebrate hox systems just happen to be the most complex and difficult of the lot? Of course not! It's mere coincidence, completely unrelated to cowardice, indolence [9], or other (entirely suppositional) defects of character. Nonetheless, even given the best of excuses, we're going to have to include some bullet points about vertebrates, just to avoid spiteful rumors.
We're going to have to weasel out here. We will not attempt to cover the vertebrates in detail. This is the Invertebrate section of Palaeos, after all. That is a reasonable excuse -- and almost credible. Can we help it if vertebrate hox systems just happen to be the most complex and difficult of the lot? Of course not! It's mere coincidence, completely unrelated to cowardice, indolence [9], or other (entirely suppositional) defects of character. Nonetheless, even given the best of excuses, we're going to have to include some bullet points about vertebrates, just to avoid spiteful rumors.
1) Vertebrates have several hox clusters: We don't mean they have a single cluster which has fragmented. Vertebrates have several, more or less complete, collinear clusters of hox genes. By convention, these are referred to as hoxa, hoxb, etc. Vertebrates have four of these clusters, except actinopterygians, which have six or more. Generally, each of the paralogous groups will be missing a few of the individual elements. The image of the Mus hox system is adapted from Carroll (2005). Variations may be found wherever fine textbooks are sold.
Some workers lept from finding multiple paralogue groups to the conclusion that the entire hox system, or even the entire vertebrate genome, was duplicated in two gigantic attacks of chromosomal schizophrenia. Amores et al. 1998); Monteiro & Ferrier (2006). We can't say that cooler heads have already prevailed, but a far more cautious approach seems to be in the wind. Chiu et al. 2004); Donoghue & Purnell (2005)*.
 2) The mouse image is ludicrously over-simplified: If you really want to know what the vertebrate hox expression system looks like, at least in the head region, look at Figure 4 in Kuratani 2005)*. Here's a direct link. Dr. Kuratani writes at least one review a year on this topic, and this is an easy way to keep up with what's going on. The equivalent of Kuratani's figure 4 has appeared in most of these reviews. It has become increasingly complex over the years. The actual hox expression patterns depend on dorsoventral level, on paralogue group, on germ layer, and on the nomadic migrations of neural crest cells as thasy drip down the sides of the embryo like streams of wax from a candle.
2) The mouse image is ludicrously over-simplified: If you really want to know what the vertebrate hox expression system looks like, at least in the head region, look at Figure 4 in Kuratani 2005)*. Here's a direct link. Dr. Kuratani writes at least one review a year on this topic, and this is an easy way to keep up with what's going on. The equivalent of Kuratani's figure 4 has appeared in most of these reviews. It has become increasingly complex over the years. The actual hox expression patterns depend on dorsoventral level, on paralogue group, on germ layer, and on the nomadic migrations of neural crest cells as thasy drip down the sides of the embryo like streams of wax from a candle.
3) Vertebrates have a large anterior hox-free zone: This zone is better known as the brain, except that hox genes are expressed in the hindbrain. Most, and perhaps all, bilaterians seem to share this hox-free brain. Arendt & Wittbrodt (2001)*. The anterior brain and sensory apparatus seems to depend on this hox-free zone. This zone is "patterned" by other homeobox genes, most notably genes of the otx and pax series. Couly et al. (2002)*; Ghysen (2003).
4) The hindbrain is a hox zone: Vertebrate hindbrain development involves a complex interaction between hox genes, mostly of the hoxb paralogy group, the "segments" of the hindbrain (rhombomeres), and the anatomically critical cranial motor nerves. The figure is based on an image from Prof. Andrew J. Waskiewicz of the University of Alberta, supplemented with information from Murakami et al. (2003)*, Carroll (2005), and Liem et al. (2001). Notice that hoxb2 is expressed anterior to hoxb1. As we've mentioned, such exceptions to anatomical colinearity are extremely rare.
The details vary somewhat among vertebrates (Murakami et al., 2003), but the general pattern is remarkably constant. Nevertheless, it is not a holdover from some primitive deuterostome segmentation pattern. The urochordates and cephalochordates show the beginnings of a vertebrate-like system of cranial nerves and midbrain structures, with some of the associated homeobox expression domains. However, neither displays hindbrain segmentation. Murakami et al. (2003); Dufour et al. 2006).
5) Vertebrate posterior hox genes are also used to mark the long axis of limbs: Hox genes are frequently re-used for other purposes, late in development. However, so far as we know, this is the only bilaterian case in which the hox system is used to mark out an axis other than the main anteroposterior axis of the body. The use of hox genes to elaborate the proximo-distal axis of limbs seems to be an accretion to the more typical limb-building toolkit involving the use of the dlx family of homeobox genes e.g. distalless in Drosophila). This is particularly interesting in that vertebrate hox and dlx also work together in the branchial/hindbrain area. These dlx genes mark the dorsoventral axis, while the hox genes "pattern" the anteroposterior axis. Carroll (2005).
The lessons from vertebrates are not clear. Does the hox system have something to do with metamerism (segmentation by generation of repeated units)? If so, what relationship? Hox genes are not known to be directly involved in segmentation in any organism. In any case, segmentation proceeds (if at all) by entirely different routes in different organisms. On the other hand, a reasonably well-developed hox system may be a necessary precondition to metamerism. It is particularly interesting that the development of multiple paralogue groups seems to coincide roughly with the development of multiple, overlapping systems of metamerism. That is, most of the vertebrate body plan (the dorsal part, at least) is based on a metameric pattern of somites, mesodermal aggregates which run down the back like a double row of buttons. However, the hindbrain and brainstem area has an apparently unrelated segmentation based on metameric rhombomeres, while the gill region is subdivided yet a third way by gill arches[10]. What about other segmented critters?
CONTINUED ON NEXT PAGE
page ATW070602
checked ATW070725
 When we get to Bilateria things are much clearer. We can triangulate back from Drosophila and vertebrates to get a reasonable idea what Urbilateria had in the way of a hox complement. Certainly it had both members of the anterior homology group, i.e. hox1 and hox2. It had at least one (and probably only one) posterior group genes. The Standard Model asserts that it had a hox3. That's a bit less clear, but still very likely. Finally, Urbilateria had a startling three central class genes. García-Fernàndez, 2005). Look back at the homology table. If those homologies are correct, this triangulation must be correct as well. Consequently, Urbilateria must have had a rather highly developed system of hox genes.
When we get to Bilateria things are much clearer. We can triangulate back from Drosophila and vertebrates to get a reasonable idea what Urbilateria had in the way of a hox complement. Certainly it had both members of the anterior homology group, i.e. hox1 and hox2. It had at least one (and probably only one) posterior group genes. The Standard Model asserts that it had a hox3. That's a bit less clear, but still very likely. Finally, Urbilateria had a startling three central class genes. García-Fernàndez, 2005). Look back at the homology table. If those homologies are correct, this triangulation must be correct as well. Consequently, Urbilateria must have had a rather highly developed system of hox genes.
 The current belief is that the gut extended all the way through the "tail" region in Vetulicolia.
The current belief is that the gut extended all the way through the "tail" region in Vetulicolia.  There seems to be a general tendency in the deuterostomes for hox1-3 to lie at some distance from hox5+, with hox4 variably placed nearer one group or the other.
See several vertebrate examples in
There seems to be a general tendency in the deuterostomes for hox1-3 to lie at some distance from hox5+, with hox4 variably placed nearer one group or the other.
See several vertebrate examples in 



