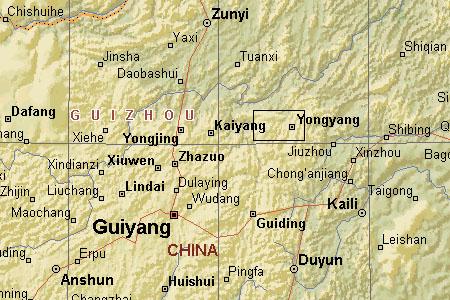
Fig. 1: Map showing approximate location of collections from Weng'an and the area to the west in central Guizhou, South China.
| Ediacaran | ||
| Neoproterozoic | Doushantuo Formation |
| Cryogenian | Mesoproterozoic | Neoproterozoic | ||
| Cambrian | Paleozoic | Timescale |
This page provides a brief overview of the Doushantuo Formation fossil beds, including geological setting, biota, and significance.
Soft-tissue fossils preserving cellular structures from the Doushantuo Formation phosphates, exposed near Weng'an in south central China, provide strong evidence for a diverse biota predating by perhaps 5 million years (Ma) the earliest of the 'classical' Ediacaran faunas from Mistaken Point, Newfoundland, and existing a good 40 to 50 million years before the Cambrian explosion (Martin et al. 2000).
Earliest reports from the Doushantuo Formation include Xiao et al. 1998, which recorded "abundant thalli with cellular structure preserved in three-dimensional detail show that latest-Proterozoic algae already possessed many of the anatomical and reproductive features seen in the modern marine flora. Embryos preserved in early cleavage stages indicate that the divergence of lineages leading to bilaterians may have occurred well before their macroscopic traces or body fossils appear in the geological record" (p. 553).
Bengtson & Zhao 1997 - Reports of fossilized eggs of marine invertebrates are rare. This may, however, largely be due to the difficulties of recognizing them. There is an abundance of small globular structures in the fossil record, including that of the Cambrian. Zhang and Pratt 1994 reported Middle Cambrian spherical fossils, 0.3 mm in diameter, that under a smooth membrane preserved a polygonal pattern which the authors interpreted as remains of blastomeres belonging to 64- and 128-cell stages of arthropod embryos. In some other cases, at least a general resemblance to eggs has been noted. We report here that two such occurrences of globular fossils from basal Cambrian rocks are eggs containing identifiable embryos of metazoans. Fig. 2A illustrates an example of the Dengying material for comparison with the Doushantuo fossils.
The documented biota now includes probable algae, sponges, cnidarians and bilaterians. Unfortunately, diagenetic effects are sometimes difficult to distinguish from genuine biological structures.
The Doushantuo phosphate deposits crop out over an area of about 57 km2 in central Guizhou (fig. 1) providing a potentially inexhaustible resource for understanding the early evolution of animal life.
 Fig. 1: Map showing approximate location of collections from Weng'an and the area to the west in central Guizhou, South China. |
In Weng'an and adjacent parts of central Guizhou, late Proterozoic sedimentary rocks are represented by the Nantuo, Doushantuo, and Dengying formations. The lowermost unit is the Nantuo Formation (~620 to 590 Ma) which unconformably overlies the middle Proterozoic Banxi metamorphic complex and is composed principally of tillite associated with the last Varanger glaciation. The overlying Doushantuo Formation, of early Ediacaran age – ~590 Ma at its base to ~565 Ma at its top, is represented by a phosphate- dolostone sequence at Weng'an, where it is 33 to 55 m thick and consists mainly of dark phosphate, cherty phosphate, chert, and gray dolomite. The overlying Dengying Formation, of Ediacaran (~565 Ma to 544 Ma) age and containing rare Ediacaran body fossils in the lower part and basal Cambrian shelly fossils (including Cloudina) near the top, is a 180 m thick dolomite sequence.
About 15 km west of the county town of Weng'an, the Doushantuo Formation consists of three units: the Lower Phosphate unit (20 m thick), the Middle Dolostone unit (3.7 m) and the Upper phosphate unit (15 m). A dark phosphate bed at the top of Lower Phosphate unit has yielded some of the richest finds reported so far.
Biomarkers from the extracts of the phosphorite samples indicate that the main sources of the organic component are non-vascular plants, protists, bacteria and archaeobacteria. Some of the biomarkers indicate a strongly reducing (oxygen-poor) depositional environment characterized by high salinity and low terrigenous input (Yin et al. 1999, p. 509).
Chemostratigraphic profiles suggest that Doushantuo fossils predate the last strongly positive C-isotopic excursion of the Proterozoic, dated as 549 ± 1 Ma in Namibia Grotzinger et al. 1995). Similarly, Doushantuo microfossils provide biostratigraphic evidence that this formation predates 555 ± 3 Ma sandstones of the Redkino Series, northern Russia, which contain diverse Ediacaran body and trace fossils. Bio- and chemostratigraphic correlations further suggest that Doushantuo fossils are older than diverse Ediacaran assemblages found in Australia, Ukraine, and northern Siberia. However, in the absence of direct radiometric constraints, it is uncertain whether Doushantuo fossils predate frondose Ediacaran remains from Newfoundland, dated as 565 ± 3 Ma, although the age of 570 Ma for the Doushantuo fossils (proposed in Martin et al. 2000 and adopted here) places them some 5 Ma earlier.
"The terminal Proterozoic follows the most recent phase of the worldwide Varanger glaciation, and it extends to the Precambrian/Cambrian boundary at 543 Ma (5, 30). In Hubei and Guizhou provinces of China, the latest Varanger glaciation event is represented by the Nantuo tillites (31). The tillites are believed to have an age of 610-590 Ma (32), and a U-Pb radiometric date suggests a greater age for the underlying formation (33). The Doushantuo Fm lies immediately above the Nantuo Fm, representing transgressive deposits that occurred as the result of a rise in sea level because of the melting of continental glaciers. The time gap between the end of the glaciation and the beginning of transgressive deposition is of unknown length, except that it is certainly younger than the 610- to 590-Ma-old Nantuo tillites. The age of the Doushantuo Fm could be as old as 580 Ma (26), and pending direct measurement, its age must fall within the range 570 ± 20 Ma (27) [Saylor et al. (34) argue that it is toward the younger end of this range]. It is important to stress, however, that whatever the absolute time horizon represented by the Doushantuo Fm, it is likely to precede the lowest strata with which bilaterian remains have so far been associated (20).
"The Doushantuo Fm is a marine deposit containing phosphate-dolomite sequences. In Beidoushan, in the Weng'an district of central Guizhou Province (Fig. 1), the phosphate deposit is divided by an erosive surface into two units. The fossils described here are from the base of the upper phosphate unit, 0.2-6 m in thickness. This high-resolution fossil bed is about 30% phosphate, present as the mineral fluorapatite [Ca5(PO4)3F]. Phosphatic beds within this deposit are grainstones composed of 1- to 5-mm phosphoclasts. These derive from a phosphatic surface that formed on the sea floor, in the process recrystallizing existing surface sediments. In addition to replacing carbonate sediments, soft tissues of metazoan embryos, larvae, adults, and algae also appear to have been mineralized (26-29). The phosphatized sediment crust was then broken into small fragments by heavy current activity and then redeposited and mixed in with adjacent lime muds. For current discussion of this fossilization process, see ref. 28." (Chen et al. 2000, p. 4457).
Analysis of the rocks and preservation of the Weng'an fossils suggests that the fossil organisms were buried alive by catastrophic sediment incursions. Phosphate alternation preserved the morphological details of the soft tissues so finely that it is possible to study the fossils at the cellular level.
Just how phosphate manages to freeze even the tiniest details of soft tissues is unclear. When the Doushantuo formed, large amounts of dissolved phosphate were apparently delivered to a shallow sea floor covered by low-oxygen waters, a place where small creatures could be preserved prior to substantial degradation.
(A) 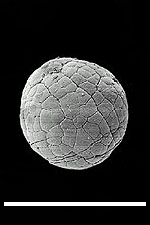 (B) (B) 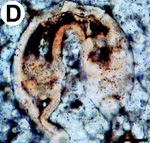 Fig. 2: (A) Reproduction of fig. 1A from Bengtson & Zhao 1997, a SEM image depicting a suggested metazoan embryo – possibly Olivooides multisulcatus – at approximately the 256-cell stage. This is a Cambrian fossil, sample NGMC (National Geological Museum of China) 9351 from the upper beds of the Dengying Formation which overlies the Doushantuo phosphates at Shizhonggou, near Kuanchuanpu village, Ningqiang County, Shaanxi, China. The scale bar is 500µm. (B) Reproduction of fig. 4D from Chen et al. 2000, an "unidentified biological form [with] a large blastocoel and a gut of some kind" photographed in thin section, as opposed to freed from the matrix as in A. The top of the fossil embryo shows slight deformation, suggesting to Chen et al. that the original structure was soft. The scale bar is 50 µm. |
The dark phosphate bed of Weng'an contains a well-preserved, diverse floral assemblage including multicellular thallophytes, acritarchs, and cyanophytes.
The associated faunal assemblage was not at first recognized: The abundant globular metazoan embryos were initially interpreted as phytoplanktonic organisms.
The specimens include tissues from a form of seaweed that has many of the cellular characteristics of modern marine plant life, and animal embryos in early as well as later stages of cell division.
Interpretation of the roughly 500µm (micrometer) spheroidal bodies as metazoan embryos containing one, two, four, eight, and more cells is supported by the observation that the fossils are the same size no matter how many cells they contain. Modern early-stage embryos behave similarly as their constant volume is divided and redivided. In contrast, algal cells tend to be similar in size, so the diameter of a clump of algal cells would depend on the number of cells in the cluster.
Some of the four-cell embryos exhibit a bilaterian tetrahedral cleavage pattern. If correctly interpreted, this finding provides further evidence that relatively modern characteristics such as bilaterality evolved before the great radiation of the Cambrian explosion.
"We want to stress that we make no claim that organisms we would recognize as polychaetes or echinoderms existed at the time the Doushantuo sediments were deposited. The comparisons with modern forms in Fig. 3 are intended only to show that the morphological characters of the fossil gastrulae are paralleled in detail by those of modern bilaterian gastrulae. Furthermore, to accommodate the diversity of the fossil gastrulae both deuterostome and spiralian models appear to be required" (Chen et al. 2000, p. 4459).
The following provides a brief review of a few selected taxa from these fossil beds.
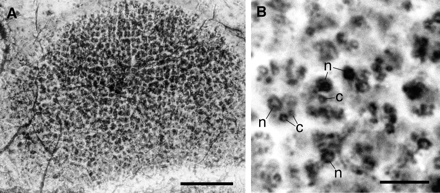 Fig. 3: (A) A thallophytic seaweed consisting of a single layer of cells, reproduced from fig. 3A of Li et al. 1998. The scale bar is 50 µm. (B) High magnification of the cells in (A), showing the nucleus (n) and chloroplast c), reproduced from fig. 3B of Li et al. 1998. The scale bar is 10 µm. |
Chen et al. fig. 3A-G
Fig. 3. Putative fossil embryos that resemble bilaterian gastrulae. (A–G) Fossils resembling deuterostome embryos; (H) Modern example (gastrulae of the sea urchin Mespilia globulus, ref. 49) In A, C, and E, the archenteron is bent to one side, and in A and C displays bilobed outpocketings; (A) The nearer ectodermal layer is thicker compared with the opposite one (possible oral and aboral ectoderms, respectively; compare H). (C) A section in the plane indicated by the small arrowheads in A.(B and D) Polarized light microscope images, showing that the cells comprising the outpocketings are differently oriented, as they appear in different colors from those constituting the walls of the gut. In A, part of the outer wall is deformed (arrow) by a crystal grain visible in B (light pink). (G), Another specimen displaying invaginating archenteron at early midgastrula stage. (H) Modern sea urchin gastrulae (49). (I and J), Fossils resembling modern spiralian gastrulae; (K) Modern polychaete embryos in which the dashed lines indicate yolky endoderm cells and dots represent mesoderm cells (Eupomatus, left; Scoloplos, right, redrawn from Anderson, ref. 50). In the fossils I and J, the archenteron is thick-walled cf. cross section in C), and in J all of the cells in the embryo, including the ectodermal wall, are conspicuously larger relative to the size of the embryo. Note also the column of cells along the archenteron in J. (Scale bars represent 50 mm.)
"We want to stress that we make no claim that organisms we would recognize as polychaetes or echinoderms existed at the time the Doushantuo sediments were
deposited. The comparisons with modern forms in Fig. 3 are intended only to show that the morphological characters of the fossil gastrulae are paralleled in detail by
those of modern bilaterian gastrulae. Furthermore, to accommodate the diversity of the fossil gastrulae both deuterostome and spiralian models appear to be
required" (Chen et al. 2000, p. 4459).
+ Critique by Xiao et al 2000
Discussion: The occurrence of sponges in the Doushantuo Formation was reported by Li et al. (1998) who claim to have identified 35 individual sponges in thin sections; mostly globular although a few tubular, and ranging in size from 150 to 750 µm. Their evidence comprises structures strongly resembling thin monaxonal spicules that are randomly dispersed in the mesohyl. The spicules survived chemical treatment by hydrochloric acid, indicating that they are siliceous. Most of the spicules are 0.5 to 1.0 µm in diameter and 10 to 60 µm long. The largest is 4 µm in diameter and 100 µm long.
Further claims, to have observed cellular-level characteristic structures of living sponges – including the epidermis, porocytes, amoebocytes, sclerocytes, and spongocoel – are far less certain.
Interpretation: Li et al. 1998 proposes that the Doushantuo sponges are monaxonid Demospongiae because their skeleton consists exclusively of siliceous and monaxonal spicules. However, it is generally interpreted that Hexactinellida evolved before both Calcarea and Demospongiae, and the recently discovered Ediacaran sponges from Mongolia (Brasier et al. 1997) are also referred to Hexactinellida.
The Weng'an sponge remains of Doushantuo age (Early Ediacaran), therefore may require revision of phylogenetic relations among the four major classes of phylum Porifera. Sponges are a monophyletic metazoan group and comprise the sister groups demosponges, hexactinellids, archaeocyaths, and calcareous sponges. Our data imply that the ancestral form in sponges lies among the demosponges.
Sponges are a major component in Lower Cambrian Chengjiang fauna. There, the skeletons in most species are represented exclusively by diactines, which form a regular, reticulate skeletal framework, and these fossils are classified as demosponges. The data from Chengjiang fauna demonstrate that main clade of early sponges, the monaxonid Demospongiae was diverse in the Lower Cambrian. Li et al. 1998 conclude that diactines evolved before other types of spicules.
Analysis of the associated rocks suggests that in the Late Proterozoic, silica biomineralization in the sponges happened in an eutrophic environment. Calcareous biomineralization in sponges (for example, archaeocyaths) is first seen in the Tommotian ~530 Ma), postdating the silica biomineralization by more then 50 million years. Calcareous biomineralization is mainly seen in oligotrophic settings. Archaeocyaths, which are possible representatives of coralline sponges, have a secondary calcareous skeleton of high Mg-calcite and are possibly derived from demosponges.
Fig. 4: Many of the flat polygonal epidermal cells have their cytoplasmic contents and nuclei preserved. Dense granules surrounding the nucleus are probably cytoplasmic organelles. Scattered among the epidermal cells are porocytes that form incurrent pores. In the mesohyl, amoebocytes with a variety of shapes were seen. In one of the best preserved specimens, six spicules were closely associated with sclerocytes. The plasma membrane of the sclerocyte is still attached to one end of the spicule. The darkly stained spongocoel has many amorphous inclusions, representing undigested debris. (A) Reproduction of fig. 1A from Li et al. 1998; a possible longitudinal section of a tubular phosphatized sponge, showing the randomly dispersed monaxonal spicules (s) in the mesohyl (m). Two spicules firmly associated with spicule-producing cells, the sclerocytes, are encircled. Scale bar is 100 µm. (B) Reproduction of fig. 1F from Li et al. 1998; two sclerocytes (sc) with their developing spicules (s) and plasma membrane (pm). Scale bar is 10 µm. (C) Reproduction of fig. 2D from Li et al. 1998; a proposed embryo at the blastula stage. Scale bar is 50 µm. |
Chen et al. embryo paper figs 2C, 2D
Fig. 2. Putative cnidarian embryos and larvae. (A) Oblique section of a possible fossil anthozoan planula. (B) Schematic view of a transverse section of the late planula of the anthozoan Euphyllia rugosa. The larval stage represented in A and B is constituted of an outer monocellular layer, the ectoderm, within which is an inner endodermal layer with various mesenteric folds and immature septa. This complicated bilayered structure is typical of anthozoan late planula larvae. Note the individual cells visible in the ectodermal layer at lower left in A, where it has separated from the endodermal layer. Scale bar, 100 mm.) (C and D) Putative fossil gastrula of hydrozoan medusa; (C) Bright field; (D) Polarized light. Under polarized light (D), both layers show the same crystal orientation at arrows, as indicated by the same colors. The modern hydrozoan embryo shown in E is Liriope mucronata. B is from Chevalier (47); E from Campbell(48). (Scale bar in Cis 50 mm.)
Chen et al. embryo paper fig 2A
Fig. 2. Putative cnidarian embryos and larvae. (A) Oblique section of a possible fossil anthozoan planula. (B) Schematic view of a transverse section of the late planula of the anthozoan Euphyllia rugosa. The larval stage represented in A and B is constituted of an outer monocellular layer, the ectoderm, within which is an inner endodermal layer with various mesenteric folds and immature septa. This complicated bilayered structure is typical of anthozoan late planula larvae. Note the individual cells visible in the ectodermal layer at lower left in A, where it has separated from the endodermal layer. Scale bar, 100 mm.) (C and D) Putative fossil gastrula of hydrozoan medusa; (C) Bright field; (D) Polarized light. Under polarized light (D), both layers show the same crystal orientation at arrows, as indicated by the same colors. The modern hydrozoan embryo shown in E is Liriope mucronata. B is from Chevalier (47); E from Campbell(48). (Scale bar in Cis 50 mm.)
Tabulate corals in the strict sense are confined to the Paleozoic, originating in the Ordovician or possibly Cambrian, and becoming extinct in the late Permian. The Doushantuo forms attributed to the Tabulata are millimeter-scale tubes that display tabulation and apical budding characteristic of the group.
Description: Tubes are circular in cross section, with a diameter of 0.1-0.3 mm; the diameter can vary markedly along the length of a single individual. No demonstrably complete tubes are known, but incomplete specimens can be more than 1 mm long. Specimens occur both as gregarious clusters with individuals oriented subperpendicular to bedding and as dispersed specimens without preferred orientation. Some tubes have thick (ca. 10-15 µm), even multilamellate outer walls (fig. 5F), likely formed or modified by diagenetic phosphatization. Others are preserved as internal molds without enveloping walls (fig. 5A). Bent specimens show folds on the compressional but not the extensional side, indicating that walls were originally flexible.
Cross-walls oriented perpendicular to the main axis divide tubes into a more or less regular series of chambers 6-12 µm thick. Most cross-walls are complete, but some tabulae extend only part of the way across the tube; they may intersect with adjacent cross-walls to form wedge-like chambers. Limited observations suggest that cross-walls are not perforated by through-going internal structures such as siphuncles.
Well-preserved specimens show that tabulae may curve slightly where they intersect with the tube wall. Indeed, well-preserved walls show that the point of insertion is thickened and wedge-like in cross section, manifested in internal molds as distinct eaves at tablet boundaries. In a few tubes, cross-walls are absent or only vaguely visible; without exception, such specimens are poorly preserved, with interiors filled by secondary silica, dolomite, or phosphatic filaments and spherules.
Terminal ends are poorly known; however, a few specimens taper to a blunt conical termination. One tube contains what appears to be a distinct terminal chamber, defined by a complete but strongly concave cross-wall. Abutting tabulations are incomplete and curved at their intersection with the chamber floor.
A few specimens show a distinctive pattern of dichotomous branching fig. 5A); tubes expand gradually along one axis to the point of dichotomy and then split into two branches, the combined circular cross sections of which are equal in area to the elliptical section below the branch point. Finally, a thin (1-µm) ridge has been observed running along the concave side of a single curved internal mold (fig. 5F); its structural or systematic importance is unclear.
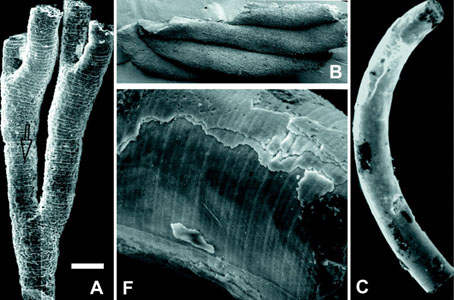
Fig. 5: Sinocyclocyclicus guizhouensis Xue et al. 1992 – Reproduction of part of fig. 3 from Xiao et al. 2000. SEM photomicrographs show (A) internal mold of branched tube; (B) four clustered tubes; (C) curved tube; and (F) enlarged view of the surface of a tube showing cross-walls and a longitudinal ridge on the concave side. Rather surprisingly, Xiao et al. 2000 does not provide locality or collection references for the figured specimens. The scale bar represents 140 µm in A, 200 µm in B, 150 µm in C, and 30 µm in F.
Further Reading
Related Pages
Other Web Sites
Bengtson, Stefan; Zhao, Yue (1997): Fossilized Metazoan Embryos from the Earliest Cambrian. Science 277: 1645-1648.
Brasier; Green; Shields (1997): Geology 25: 303.
Chen, Jun-Yuan; Oliveri, Paola; Li, Chia-Wei; Zhou, Gui-Qing; Gao, Feng; Hagadorn, James W.; Peterson, Kevin J.; Davidson, Eric H. (2000): Precambrian Animal Diversity: Putative Phosphatized Embryos from the Doushantuo Formation of China. Proceedings of the National Academy of Sciences of the USA 97: 4457-4462.
Grotzinger, J.P.; Bowring, Samuel A.; Saylor, Beverly Z.; Kaufman, Alan J. (1995): Biostratigraphic and Geochronologic Constraints on Early Animal Evolution. Science, 270: 598-604.
Li, Chia-Wei; Chen, Jun-Yuan; Hua, Tzu-En (1998): Precambrian Sponges with Cellular Structures. Science v. 279, issue of 6 February 1998, pp. 879 - 882.
Martin, M.W.; Grazhdankin, D.V.; Bowring, S.A.; Evans, D.A.D.; Fedonkin, M.A.; Kirschvink, J.L. (2000): Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution. Science v.288: 841-845.
Xiao, Shuhai; Zhang, Yun; Knoll, Andrew H. (1998): Three-Dimensional Preservation of Algae and Animal Embryos in a Neoproterozoic Phosphorite. Nature 391: 553-558.
Xiao, Shuhai; Yuan, Xunlai; Knoll, Andrew H. (2000): Eumetazoan Fossils in Terminal Proterozoic Phosphorites? Proceedings of the National Academy of Sciences of the United States, 97 (25): 13684-13689.
Yin, Chungu; Zhang, Yun; Jiang, Naihuang (1999): Organic Matters from the Fossil-Bearing Phosphorites of the Neoproterozoic Doushantuo Formation in Guizhou, South China. Peking University Geology and Goegraphy, 35 (4): 509-517.