
| Palaeos: |  |
Bones: Teeth |
| Vertebrates | Overview-3 |
| Page Back | Unit Back | Unit Home | Unit Dendrograms | Unit References | Glossary | Taxon Index |
| Page Next | Unit Next | Vertebrates Home | Vertebrate Dendrograms | Vertebrate References | Bones | Time |
|
Bones
|
Teeth
|
Thus , the conodont apparatus does not seem to be made of odontodes as they are understood today. Nevertheless, at least the dentine cone part of the odontode seems to have appeared very early in vertebrate evolution, possibly even before the vertebrates themselves. Isolated scales of this type are known from the Furongian (Late Cambrian), and the typical dentine scale cone is commonplace among both the Pteraspidomorphi and Thelodonti. The evolution of hard tissues in pteraspidomorphs was quite diverse, and the development of the mineralized integument here is still obscure. Halstead [T64] makes a compelling case that the external dentine-capped tubercles were replaced and/or repaired throughout adult life. In order to explain this phenomenon, he invokes a metabolic connection through the supposedly acellular aspidine matrix. This work is discussed elsewhere. The point (also made by Halstead) is that, as in conodonts, this required periodic covering of the surface with some epidermal tissue. However, in this system, mesenchymal tissue was presumably proliferating below the epidermis, creating something like familiar dentine odontode structure with its array of dentine canals.
Assuming
the conodont process to be basal, it is natural to suppose that the epithelium
could be engaged to apply a thin layer of acellular hypermineralized tissue to
the surface. The literature is full of confusion and
contradiction about whether an "enamel" layer was sometimes present on
the outer surface of heterostracan plates and scales. Halstead [T64] denies this
was present in psammosteids,
and we have no reason to doubt him. However he also supplies some
interesting comparative images of other heterostracans plates, one of which (Tesseraspis)
is
reproduced here. Note that the right and left halves show the same image,
but the image on the right is taken with "crossed nichols." In
this technique, light is passed through a polarization filter before reaching
the specimen. Another filter, oriented at 90° to the first, is placed
between the specimen and the camera. 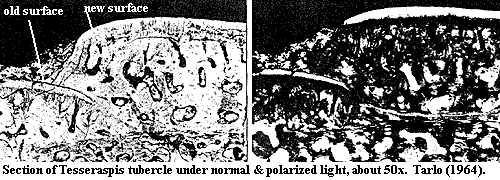 Light can only reach the camera if
the light waves are rotated by the specimen. Bright regions then probably
indicate crystals capable of rotating the light waves through some consistent
angle.
Light can only reach the camera if
the light waves are rotated by the specimen. Bright regions then probably
indicate crystals capable of rotating the light waves through some consistent
angle.
In Halstead's image, there are two types of apparently crystalline materials: "lines" and shapeless blobs. The blobs correspond to vacuities in the dentine and are probably due to calcite or some other post-mortem infilling. The lines, however, are clearly dense material on the surface of both new and partially overgrown tubercles. Note especially the tubercle labeled "old surface." Here, we can observe dentine tubules around which the apatite has been partially resorbed. The tubules appear to hang down like hairs from the surface material, but they do not enter it. The tubule region is dark and probably amorphous and non-crystalline. By contrast, the surface material shines brightly, and tubules are absent. In short, we have ordered, dense, crystalline material on top of dentine tubules -- a very good indicator of an enamel-like material, without evidence of inclusions from osteoblasts. The enamel appears to be perhaps 20-30 µ thick -- thin, but much more substantial than the surface coating in conodonts. Thus, our best guess is that these are ordered apatite crystallites. The presence of some long-distance ordering should give us pause. It strongly suggests that the crystals did not form randomly, but were lined up on a regular matrix.
Does this mean that true enamel is a synapomorphy of the vertebrates? No. However, it does seem certain that the heterostracans had achieved the basic tissue configuration needed to use one of the most powerful utensils in the vertebrate developmental toolbox, the epithelial - mesenchyme interaction discussed earlier. In fact, those interactions would be hard to avoid. If Halstead is right, then during periods of plate repair, mesenchyme would proliferate from scattered dormant refugia in the aspidine and grow in the gaps between tubercles and wherever else there was room to grow. At the same time a thin epithelium would grow over the plates or scales to protect the mesenchyme. The two tissues would meet precisely at those places where the dentine tubercles had been damaged or abraded away. Thus, it would be natural, even unavoidable, that this contact would become the metabolic signal for the mesenchyme to produce dentine tubercles. It would be equally natural, although perhaps not unavoidable, that the epithelium would evolve a method to produce a hypermineralized "finish" to seal off the external surface of the tubercles. The physical evidence suggests that this layer was thick enough, and ordered enough, at least in some cases, to infer the presence of some protein matrix. However heterostracan "enamel" is not thick enough to be true enamel, nor does it have the multi-layered appearance of a mixed collagen-amelogenin enameloid system. What we are probably seeing is a single-layered collagen based matrix -- similar to the conodont system, but ordered by specialized epithelial cells, rather than simply painted on top in some random orientation.
In the thelodonts,
we see several critical advances. Thelodont scales presumably developed
subcutaneously. This seems a small matter, but it meant that the fish no
longer had to undergo periodic repair cycles and could remain active at all
times. Furthermore, scales would be produced at a permanent epithelial -
mesenchyme boundary, which allowed the formation of regular scales of a
distinctive shape and pattern. That shape could then evolve to become more
hydrodynamic, improving mobility.
 |
|
Comparison of thelodontid and loganiid scales. The thelodontid scale is relatively small and has a rounded crown. It has a more distinct pulp cavity with a uniformly sharp border. The dentine tubules and canals are generally straight and there are relatively few spaces (trapped cell bodies) in the crown. This histology is characteristic of orthodentine. The base is slightly inflated and (although difficult to see in this view) tends to form a toroidal opening to the pulp cavity. The loganiid scale is larger and has a rather flat crown. The pulp cavity is barely discernable, and its margin is rough or indistinct in the central region. The dentine tubules form a messy-looking network with numerous spaces. This histology is characteristic of mesodentine. The two examples are both from Karatajute-Talimaa & Marss (2002). |
The hypermineralized cap remained very thin, although the outer surface often became very elaborately ornamented. This suggests that the epithelial interaction with the base odontode was becoming increasingly sophisticated. Thelodonts also produced several different types of dentine, as shown in the image above. This variety tends to confirm the widespread suspicion dentine histology has rather limited phylogenetic value.
Far more revealing are the long-range antero-posterior changes in the scale morphology, as shown below.
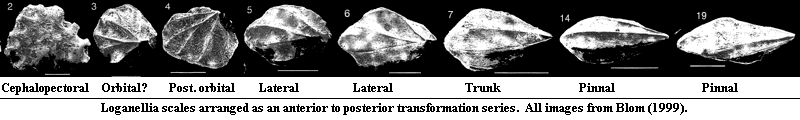
This transformation series has been somewhat artificially assembled. However, it is sufficient to illustrate two differences from the heterostracan condition, each of which has specific developmental implications.
 Specific center of symmetry: Rather than some sort of
vaguely pustule shaped dome, thelodont scales have one or more specific centers
of symmetry. This probably correlates with an organizing center or
"enamel knot." This, in turn, suggests the presence of an Shh
pathway using the same BMP4-dependent signaling system discussed
above.
Specific center of symmetry: Rather than some sort of
vaguely pustule shaped dome, thelodont scales have one or more specific centers
of symmetry. This probably correlates with an organizing center or
"enamel knot." This, in turn, suggests the presence of an Shh
pathway using the same BMP4-dependent signaling system discussed
above.
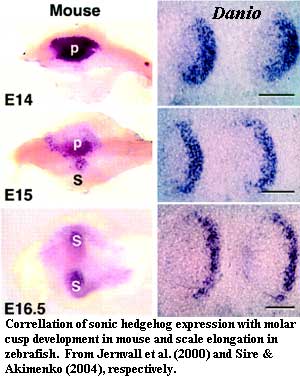
Movement of the Axis: Further, the center of symmetry moves posteriorly in a rather regular sequence. In both the teeth of mammals and the elasmoid scales of most living actinopterygians, directional "stretching" of the tooth or scale is associated with a specific pattern of Shh expression, late in development, in which Shh expression spreads out from the primary "enamel knot" into subsidiary cusps (tooth) or along a broad growth front (scales) [SA04]. This phylogenetic bracket (assuming we've got the phylogeny right) allows us to postulate that thelodont scale development had begun to be governed by the same mechanism.
But two things are also missing:
No dorsoventral patterning: Thelodont scales show very little dorsoventral patterning. It is generally not possible to tell whether a body fossil is in dorsal or ventral orientation. See, for example the famous debate over the holotype of Turinia [DS01].
No prepatterning: in osteichthyans, scale development proceeds from an initiation scale in regular rows, and the epidermis appears to be prepatterned in some manner to produce scales only in certain positions [SA04]. In thelodonts, the scales show no signs of a regular arrangement. There are two known exceptions, both in highly derived thelodonts. Lanarkia has two kinds of scales. The large, "trumpet" scales are arranged at regular intervals in lines running down the body parallel to a mid-dorsal line of ridged scales. This is interesting, but not particularly informative, as the mid-dorsal ridge line is easily explained by the unique relationship to the notochord. While we cannot know the molecular biology of thelodonts, the notochord is a notorious producer of Shh, thus providing a ready explanation for strange effects in this area which do not require pre-patterning. The more interesting exception occurs in Loganellia.
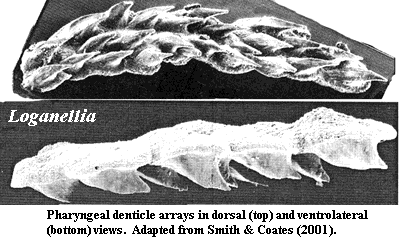 The more important example for present purposes are the branchial denticle arrays found
in some thelodonts [vJ93] [SC01]. Or, to be more precise, they are found in Loganellia
-- one of the thelodonts which is most gnathostome-like in other respects.
Loganellia did not have jaws, but it did have gill arches, and
these are where the denticle arrays are believed to have come from. Jaws
are quite probably related to the gill arches, and pharyngeal teeth associated
with the gill arches are very common in living actinopterygians. See, for
example, the elaborate gill arch dentition of Amia
or the Ostariophysi. Similar
pharyngeal denticle arrays are found in the pharynx of the early chondrichthyan,
Stethacanthus
(a/k/a Akmonistion),
and in some acanthodians
[SC01]. Most recently, similar structures have been reported from the
branchial chambers of all major placoderm groups [JS03] [1].
The more important example for present purposes are the branchial denticle arrays found
in some thelodonts [vJ93] [SC01]. Or, to be more precise, they are found in Loganellia
-- one of the thelodonts which is most gnathostome-like in other respects.
Loganellia did not have jaws, but it did have gill arches, and
these are where the denticle arrays are believed to have come from. Jaws
are quite probably related to the gill arches, and pharyngeal teeth associated
with the gill arches are very common in living actinopterygians. See, for
example, the elaborate gill arch dentition of Amia
or the Ostariophysi. Similar
pharyngeal denticle arrays are found in the pharynx of the early chondrichthyan,
Stethacanthus
(a/k/a Akmonistion),
and in some acanthodians
[SC01]. Most recently, similar structures have been reported from the
branchial chambers of all major placoderm groups [JS03] [1].
Note that these denticles are fused at the base and form roughly linear arrays. The appearance of ordering on the gill arches, but not in the integument, suggests that this patterning is related to the influence of pharyngeal endoderm. The influence of endoderm is often said to be essential to the formation of teeth [DS02]. This is, at least, subject to exceptions. See the discussion of Denticeps, below. However, sensitivity to endoderm strongly suggests the integration of the odontode development system with endodermal transcription factors, such as Pax9.
The implication here is that this linear aggregation of denticles is a key synapomorphy joining gnathostomes and Loganellia. This may be the case. Both Loganellia and Sheilia, another Loganiid, have been reported to possess multiple gill slits and pectoral fins. Sheilia may even have pelvic fins, specialized scales on the fin leading edges and some degree of dorsoventral scale specialization [M+02].
We are now at last ready to discuss the gnathostomes and the subject of teeth. Unfortunately, at this key transition point we are also about out of things to say to which we can attach much confidence. The evo-devo of the jaw is perhaps as poorly understood as the gross morphological change it underlies. This particular question has inspired a number of inspired guesses. A few years ago a group of molecular scientists, reinforced by Philippe Janvier, nominated Otx1 as a key inducer based on its presence in a number of characteristic gnathostome or near- gnathostome features [M+00]. In particular, Otx1 is expressed in the horizontal semi-circular canal of the middle ear, which evolved at about the same phylogenetic level as the jaw. However, Otx1 seems to be more or less confined to neural and brain-related tissues [Z+02] and no information to date relates it to tooth, jaw or scales. Other possible candidates might include some member of the Wnt series, a novel BMP signal, or perhaps a mutant Shh.
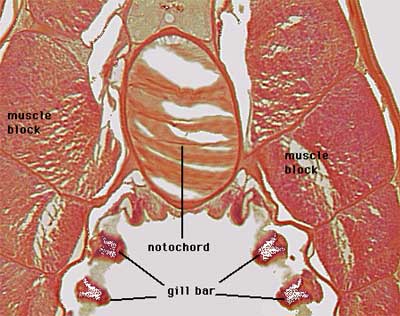 Our personal endorsement
might go to something more along the lines of Pax9. The Pax
family of genes code for proteins with a highly conserved, 128-amino acid, N-terminal
paired DNA binding domain
[H+00], an octapeptide of unknown function, and a homeobox domain. The C-terminal portions of the protein are rather variable. Like many vertebrate transcription
factor genes, the Pax genes come in pairs [2]. Pax1
and Pax9 are quite similar, and both lack the homeobox domain region. The extant cephalochordate
Branchiostoma has only one gene, which appears to be homologous to both Pax1
and Pax9 [H+00]. This gene, AmphiPax1, is expressed in the gill
bars [H+00], homologous to
the inner, endodermal gill pouches and (later) gill arches of vertebrates
[L+01]. Pax9 is similarly expressed in the gill arches of
vertebrates (and, it seems, in
the inner ear). It is also expressed, largely in the endoderm, in a
variety of other phylogenetically interesting places, such as the developing
vertebrae [P+99], limb buds, and -- of course -- the jaw.
Interestingly, it is not expressed in the axial "skeleton" of
non-vertebrate chordates [DS02]. Given the
developmental information we have, and the phylogenetic information we have
inferred, one crucial test of Pax9 is whether its expression is related
to Shh expression. As it happens, both Pax1 and Pax9
are initially induced by Shh, from the notochord (for vertebrae) [P+99],
presumably from the apical ectodermal ridge (limbs), the neural crest
ectomesenchyme (for pharyngeal arches), and the enamel knot (for teeth).
We understand (i.e. from abstracts only) that Pax9 also induces BMP4
production, which modulates Shh expression in teeth as discussed
earlier. Thus it seems likely that some relatively complex feedback
control mechanism is involved.
Our personal endorsement
might go to something more along the lines of Pax9. The Pax
family of genes code for proteins with a highly conserved, 128-amino acid, N-terminal
paired DNA binding domain
[H+00], an octapeptide of unknown function, and a homeobox domain. The C-terminal portions of the protein are rather variable. Like many vertebrate transcription
factor genes, the Pax genes come in pairs [2]. Pax1
and Pax9 are quite similar, and both lack the homeobox domain region. The extant cephalochordate
Branchiostoma has only one gene, which appears to be homologous to both Pax1
and Pax9 [H+00]. This gene, AmphiPax1, is expressed in the gill
bars [H+00], homologous to
the inner, endodermal gill pouches and (later) gill arches of vertebrates
[L+01]. Pax9 is similarly expressed in the gill arches of
vertebrates (and, it seems, in
the inner ear). It is also expressed, largely in the endoderm, in a
variety of other phylogenetically interesting places, such as the developing
vertebrae [P+99], limb buds, and -- of course -- the jaw.
Interestingly, it is not expressed in the axial "skeleton" of
non-vertebrate chordates [DS02]. Given the
developmental information we have, and the phylogenetic information we have
inferred, one crucial test of Pax9 is whether its expression is related
to Shh expression. As it happens, both Pax1 and Pax9
are initially induced by Shh, from the notochord (for vertebrae) [P+99],
presumably from the apical ectodermal ridge (limbs), the neural crest
ectomesenchyme (for pharyngeal arches), and the enamel knot (for teeth).
We understand (i.e. from abstracts only) that Pax9 also induces BMP4
production, which modulates Shh expression in teeth as discussed
earlier. Thus it seems likely that some relatively complex feedback
control mechanism is involved.
Unfortunately, more recent (i.e., 2001 and later) work has uncovered whole new groups of transcription factors which appear to be involved in molding the characteristic structures of gnathostomes: Dlx, and Lef genes as well as novel FGFs and Msxs [J+03]. Thus we must retire in disorder from this otherwise entertaining subject. However, if we were forced at gunpoint to speculate, we might choke out the words "sonic hedgehog."
Shh itself has not changed, of course. It is more or less the same gene in all metazoans. What seems to have happened is that Shh somehow became capable of directing traffic along induction pathways on which it was previously a mere pedestrian. It has always had the ability, as we saw in scale development, to alter polarity and shape. Why does it suddenly become a key inducer in creating a variety of new shapes and structures? A likely mechanism is not at all hard to identify: the profligate use by vertebrates of ectomesenchyme from the neural crest, combined with the equally profligate creation, discussed above, of new transcription factor paralogues during early vertebrate evolution.
Neural crest ectoderm (or ectomesenchyme) is the hallmark of the chordates. As the chordate head and brain develop, this material drips down from the dorsal neural crest over the rest of the body like water overflowing a bathtub. It comes in contact with all kinds of tissues and enters into the formation of all of the characteristic vertebrate -- and gnathostome -- structures. In particular, it forms the entire splanchnocranium (gill arches, jaws and related structures) [DS02]. Shh is involved at every step, usually through its ability to interact with bone morphogenic proteins (BMPs), as mentioned above. Shh is secreted by the neural plate and notochord during formation of the neural crest tissue, and also helps mediate cell adhesion during neural crest migration [T+01]. It is deeply involved in differentiation and, especially, morphogenesis of all of the structures of interest, again frequently through its ability to play nicely with BMPs.
Now, one developmental "problem" of early vertebrates would be to evolve a mechanism for managing the enormously complex morphology of these novel structures. Fortunately, the early vertebrates had handy two to four paralogues of many transcription factors thanks to the gene duplication events previously discussed. In order to control the shapes of the structures controlled by the pathways influenced by these factors, it would be a simple, and practically inevitable, matter to develop a mechanism by which one paralogue reacted differently than the other to either Shh or to BMPs controlled by Shh. By natural variation in the degree of Shh/BMP sensitivity, the amount of the transcription factors present, and the preexisting Shh/BMP interactions, practically any desirable form could be evolved in relatively short order.
Thus, quite without meaning to, we have blundered into a testable theory of gnathostome evo-devo. It should be possible to show that the paired Pax, Otx, Lef, Msx, and (particularly!) Dlx paralogues show a diverging pattern of pattern of responses to Shh/BMP regulation over the course of vertebrate evolution. This is really only a molecular variation on Williston's Law. Furthermore, we should look even more closely at molecular development in those rare vertebrates, such as the Chondrostei, which have clearly undergone additional rounds of polyploidy (genome replication) during more recent geological time. Of course, the fact that that a hypothesis is testable does not mean it is right; but we can, at least, look forward to day when we may be proven wrong.
Continued on Next Page
| Page Back | Unit Home | Glossary | Page Top | Page Next |
checked ATW030403
all text placed in the public domain