
| Palaeos: |  |
Bones: Teeth |
| Vertebrates | Overview-4 |
| Page Back | Unit Back | Unit Home | Unit Dendrograms | Unit References | Glossary | Taxon Index |
| Page Next | Unit Next | Vertebrates Home | Vertebrate Dendrograms | Vertebrate References | Bones | Time |
|
Bones
|
Teeth
|
Having completed this sojourn through the arcana of molecular embryology and petrified fish scales, we may return to the subject with which we began, namely the peculiar theoretical constructs which make up "scale theory." We will begin with the dominant scale theory of the mid-Twentieth Century. Despite its numerous failings, it is still mindlessly repeated in many texts. Thus, the well-prepared student still needs to know this enemy and arm himself to extirpate any remaining germs of its false doctrine. We will then modulate to a more harmonic scale, the odontode theory of Reif, as practiced by various paleontologists today. Finally, we will bang away at various points, in a manic fashion, to demonstrate certain dissonances which may be inherent in the whole idea of a scale theory.
 As discussed briefly elsewhere, scale theory in the middle
reaches of the last century meant the Scandinavian School, of which Erik
Stensiö was the best-known and most vocal proponent. This was a
typological theory which attempted to explain all scales and teeth as simply
differing combinations of theoretical scale units called lepidomoria.
A lepidomorium is essentially the three-part unit we have been discussing from
the beginning, consisting of bone base, a dentine-covered pulp cavity, and a
hypermineralized cap. However, the Scandinavian School also supposed that
the structure was fundamentally circumscribed by a single vascular loop as shown
in the image. Thus, in this view, essentially all real scales and teeth
represent coalescences of these theoretical units [D02]. As Donoghue [D02]
points out, the lepidomorial theory is a "concrescence" based
model. Scales and teeth only recombine lepidomoria in different
ways. The basic lepidomorial unit never evolves or differentiates.
Despite this peculiar, almost anti-evolutionary way of looking at things, the
theory was enormously influential.
As discussed briefly elsewhere, scale theory in the middle
reaches of the last century meant the Scandinavian School, of which Erik
Stensiö was the best-known and most vocal proponent. This was a
typological theory which attempted to explain all scales and teeth as simply
differing combinations of theoretical scale units called lepidomoria.
A lepidomorium is essentially the three-part unit we have been discussing from
the beginning, consisting of bone base, a dentine-covered pulp cavity, and a
hypermineralized cap. However, the Scandinavian School also supposed that
the structure was fundamentally circumscribed by a single vascular loop as shown
in the image. Thus, in this view, essentially all real scales and teeth
represent coalescences of these theoretical units [D02]. As Donoghue [D02]
points out, the lepidomorial theory is a "concrescence" based
model. Scales and teeth only recombine lepidomoria in different
ways. The basic lepidomorial unit never evolves or differentiates.
Despite this peculiar, almost anti-evolutionary way of looking at things, the
theory was enormously influential.
However, no one has ever found a true lepidomorium. The theory could be used to describe any scale or dental structure, but it was unclear that it actually explained anything. Thus, by the 1990's, the Scandinavian School had few partisans -- but also few serious challengers. One of those few was the extraordinary [3] Prof. Wolf-Ernst Reif of the University of Tübingen. In about 1976, Reif began an empirical study of the squamation and dentition of sharks which lasted almost twenty years. What Reif found was difficult to reconcile with the approach taken by Stensiö and others of the Scandinavian School. Lepidomorial theory predicted that scales and teeth were simply slightly different arrangements of the same basic unit. In sharks such as Scyliorhynchus, in which both structures are very simple, one might expect that teeth and scales would be developmentally integrated. They're not. See, e.g., [R80]. The sequence and timing of development are quite different, even in sharks with oral denticles other than teeth. Scales and teeth do not grade into each other. Teeth develop as families, while scales develop individually. The bone of attachment is derived quite differently.
 Odontode Differentiation Theory
Odontode Differentiation TheoryWhat Reif proposed was a very different approach. First and foremost, he substituted the odontode -- a real structure found in real organisms -- for the theoretical lepidomorium. Although he still regarded the odontode as a sort of fundamental unit, Reif argued that odontodes are capable of differentiating into various types and evolving in more or less the same way as any other biological structure. As Donoghue [D02] points out, this is the very opposite of the lepidomorial theory, with its supposed integration of invariant fundamental units. Odontodes may physically merge, but they evolve by differentiation of different types of the fundamental unit. Reif, as summarized by [J96] and [D02], posits that odontodes are organized with respect to each other by a "zone of inhibition" of greater or lesser size and persistence, which inhibits the formation of other odontodes in the immediate vicinity of an existing structure (this is another example of the Sultan effect, discussed elsewhere). Donoghue [D02] argues that this kind of spatial organization is accomplished by co-expression of Shh, one or more BMPs and FGF4, as in the enamel knot of the developing mammalian tooth. The BMPs are Shh antagonists, but are also low molecular-weight substances which diffuse outward rapidly. Thus, the region around the source is rich in the FGF4, a promoter of Shh, while surrounding areas have a preponderance of BMPs, suppressing the formation of competing centers of Shh activity.
Dr. Moya Smith and others have argued cogently that tooth and scale, although presumably derived from the same source, diverged long ago. See, e.g., [SC01]. Pressing this phylogenetic analogy, they assert that teeth and scales separated at a very early point in vertebrate phylogeny and have specialized in quite different ways [SC01][D02]. In a long series of papers, Smith and co-workers have developed a great deal of new information on the evolution of endoderm-influenced denticles.
 Now
that we have walked our way through the basic chords of scale theory, we may
explore some of its underlying problems. Actually, in our view, it has
only one failing, although it is a glaring flaw. That flaw is an inheritance
from the lepidomorial theory: the assumption that the odontode is an irreducible
and fundamental unit of development, evolution and homology. That's a
nice, rolling sentence, but what does it mean?
Now
that we have walked our way through the basic chords of scale theory, we may
explore some of its underlying problems. Actually, in our view, it has
only one failing, although it is a glaring flaw. That flaw is an inheritance
from the lepidomorial theory: the assumption that the odontode is an irreducible
and fundamental unit of development, evolution and homology. That's a
nice, rolling sentence, but what does it mean?
Look at it this way. One difficulty with the odontode specialization model is that it rests on an inexact analogy. The development of structures in organisms is like the evolution of the whole organisms. But the process is not the same. To the extent that oral and dermal odontodes are developed in the same fashion, they depend on the same set of developmental signals and, more generally, the same gene products. A change in any of these genes will affect both processes, at the same time, and very often in the same way. So, for example, the initial development of odontodes seems to depend on a very complex and stylized set of two-way epithelial - ectomesenchyme interactions mediated by BMP4 and FGF8, among others [G00]. Any change in any of these genes, or the receptivity of common tissues to these signals, will alter both types of odontodes. Thus teeth and scales have not "diverged," "differentiated," or "specialized" in quite the same way as we might use those words to describe the evolution of two species. Two species are, by definition, no longer in genetic contact. Teeth and scales still use many of the same genetic programs in the same genome.
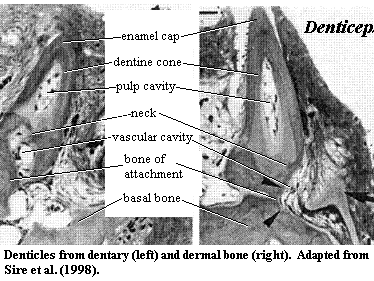 Consider
a concrete example. As Donoghue & Sansom [DS02] point out,
one problem case comes in the
unlikely form of a particularly ugly Nigerian clupeomorph
fish which was not discovered until the 1950's. This fish, Denticeps
clupeoides, and a few of its close relatives, have apparently reversed the
process of turning scales into teeth. That is, the teeth of Denticeps
have escaped the jaws and pharyngeal arches and appear as isolated denticles on
the surface of the dermal bones [S+98]. The result is a structure
quite similar to that found in the armored catfishes and a few other teleosts,
in the coelacanth [S+98], and
perhaps in the more basal sarcopterygian, Grossius
[S73]. The oral and dermal denticles have almost the same morphology
and ultrastructure. In short, the teeth of Denticeps work
perfectly well as scales, and it would not be surprising to find that different
sorts of odontodes have crossed these functional borders at numerous
times. Indeed, many workers believe that the scales of extant teleosts are
derived from teeth, rather than from the scales of their distant ancestors
[SA04].
Consider
a concrete example. As Donoghue & Sansom [DS02] point out,
one problem case comes in the
unlikely form of a particularly ugly Nigerian clupeomorph
fish which was not discovered until the 1950's. This fish, Denticeps
clupeoides, and a few of its close relatives, have apparently reversed the
process of turning scales into teeth. That is, the teeth of Denticeps
have escaped the jaws and pharyngeal arches and appear as isolated denticles on
the surface of the dermal bones [S+98]. The result is a structure
quite similar to that found in the armored catfishes and a few other teleosts,
in the coelacanth [S+98], and
perhaps in the more basal sarcopterygian, Grossius
[S73]. The oral and dermal denticles have almost the same morphology
and ultrastructure. In short, the teeth of Denticeps work
perfectly well as scales, and it would not be surprising to find that different
sorts of odontodes have crossed these functional borders at numerous
times. Indeed, many workers believe that the scales of extant teleosts are
derived from teeth, rather than from the scales of their distant ancestors
[SA04].
Here's the issue: scale theory assumes that the odontode is the relevant unit for this analysis. It isn't. An odontode is a type: a shorthand for a series of developmental and genetic processes which often occur together and are manifested in a typical morphology. The odontode has shown a lot of stability over the last 500 My -- much more stability than most organs -- but that should not mislead us into seeing it as any more than a type. It is no more fundamental or indivisible than the fin/arm/flipper/wing or the gill arch/jaw/auditory ossicle. All of the parts of a typological odontode may be present, or they may not. We have discussed many scales which lack the enamel cap, or where the hypermineralized portion is produced in quite disparate ways. On the other hand, scales, teeth and other integument derivatives may all be affected in a coordinate fashion by genetic changes in the developmental programs which they still have in common. It is often useful and convenient to speak in terms of the odontode unit, but it is a typological fiction we should be willing to abandon the moment it ceases to be the real issue. With our rapidly increasing knowledge of the molecular underpinnings of integumentary structures, our willingness needs to all the greater. ATW040709
[1] But what about the (randomly placed) oral denticles and (linear, ordered) external mouth scales of some cyathaspidiforms, the apparently linear arrays of external scales in the Corvaspididae, or the serial spines of the conodont apparatus? Unlike the thelodont branchial denticles, these examples probably all have reasonable explanations which do not require us to invoke any evolutionary novelties. However, they also suggest caution.
[2] The tendency of many vertebrate regulatory genes is to come in groups of at least two paralogues. This is said to be the result of very broad gene duplication events which seem to have affected many regulatory genes, and perhaps the entire genome. There were probably at least two distinct duplications which occurred in the passage from early chordates to the early vertebrates [H+00].
[3] We do not use the word lightly. Prof. Reif's name will never be a household word, but his contributions to theoretical biology (and other fields) have been, and continue to be, as remarkable as they are varied.
[4] The reader is warned that this approach is heterodox.
[5] We adhere to the convention of denoting genes in italics, with the corresponding gene products in normal typeface. Thus, the "Shh (gene) was active" but the "Shh (transcription factor) was present." In many cases, we are not certain whether the species exported, or even the active species, is an RNA or a protein coded for by an mRNA. For our purposes, it rarely makes a difference. Accordingly, all gene products are in normal typeface.
[6] This specificity is true of all known gnathostome systems. The migration of neural crest in lampreys appears to be less tightly constrained [MB03].
 [7]
Dzik [D00] argues that the conodont elements were continuously covered
with both secretory epithelium and a keratinous ("horn") layer.
However the images of both Dzik and of Donoghue & Purnell [DP99] strongly
support discontinuous growth, and it is difficult to see how a thin layer of
secretory cells could be maintained -- mechanically or metabolically -- under a
keratin sheath. Dzik offers arguments based on the morphology of the cell
outlines. The outlines are clearly elongated, with thinner lines of
crystals, in locations in which the slope changes rapidly, suggesting that this
reflects a slower growth rate. The argument is ingenious, but may confuse
cause with effect. The shape of the surface would naturally create such a
pattern if it were brought into contact with a taught, flat, elastic epithelium,
approaching at a slight angle.
[7]
Dzik [D00] argues that the conodont elements were continuously covered
with both secretory epithelium and a keratinous ("horn") layer.
However the images of both Dzik and of Donoghue & Purnell [DP99] strongly
support discontinuous growth, and it is difficult to see how a thin layer of
secretory cells could be maintained -- mechanically or metabolically -- under a
keratin sheath. Dzik offers arguments based on the morphology of the cell
outlines. The outlines are clearly elongated, with thinner lines of
crystals, in locations in which the slope changes rapidly, suggesting that this
reflects a slower growth rate. The argument is ingenious, but may confuse
cause with effect. The shape of the surface would naturally create such a
pattern if it were brought into contact with a taught, flat, elastic epithelium,
approaching at a slight angle.
[8] Or alternatively, as stated by [D02], BMP2 and/or BMP4 inhibit the ability of FGF8 to induce Pax9 expression.
| Page Back | Unit Home | Glossary | Page Top | Page Next |
checked ATW050517
all text placed in the public domain