
| Palaeos: |  |
Bones: Teeth |
| Vertebrates | Overview |
| Page Back | Unit Back | Unit Home | Unit Dendrograms | Unit References | Glossary | Taxon Index |
| Page Next | Unit Next | Vertebrates Home | Vertebrate Dendrograms | Vertebrate References | Bones | Time |
|
Bones
|
Teeth
|
The purpose of this somewhat tedious essay is to unite and expand on a number of themes discussed in other parts of Palaeos. In particular, this discussion follows up some obscure hints dropped in our treatment of corvaspid scales. There we made various disparaging remarks about the Scandinavian School of "scale theory," and predicted that it was nearly hopeless to try and dig out of the dark scientific hole in which the Scandinavian School had left us. Since then, we have encountered the recent work of Philip Donoghue, Jean-Yves Sire, and others. Whether they will dig us out is hard to say, but they are certainly wielding their shovels with tremendous energy. All this furious activity has briefly roused us from despondent lethargy to toss a little sand about, as well.
 What's
at stake here is just about everything that a vertebrate uses to meet the
environment except bare skin. Unlike
black holes, vertebrates have hair -- and feathers and scales and teeth and
so on. Over the last 500 million years or so, the vertebrates have come up
with a fair selection of bits and pieces which stick out of the skin. The structure and development of all of these fashion accessories has many common features
-- so many that it is virtually certain that they all derive from a common
source [W+04]. Here, we will focus on the evolution of that system in body
scales (presumably the system in which the whole business first evolved) and
the way it was adapted to form teeth.
What's
at stake here is just about everything that a vertebrate uses to meet the
environment except bare skin. Unlike
black holes, vertebrates have hair -- and feathers and scales and teeth and
so on. Over the last 500 million years or so, the vertebrates have come up
with a fair selection of bits and pieces which stick out of the skin. The structure and development of all of these fashion accessories has many common features
-- so many that it is virtually certain that they all derive from a common
source [W+04]. Here, we will focus on the evolution of that system in body
scales (presumably the system in which the whole business first evolved) and
the way it was adapted to form teeth.
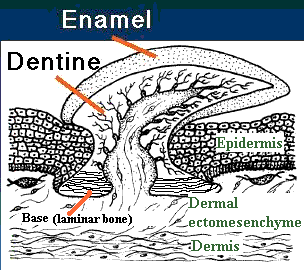
Teeth and early vertebrate scales are generally treated as arising from a fundamental unit, the odontode or lepidomorium. The differences between these two (theoretical) structures will be discussed in due course. For the moment, we will speak of the odontode as it is usually discussed. The basic odontode unit is identical to the placoid scale of chondrichthyans [SA04] [S+98]. The odontode has three parts: (a) a dentine-covered cone with an internal pulp cavity (b) attached to a base of laminar bone or cartilage, and (c) capped by hypermineralized tissue [S+98], often loosely referred to as "enamel."
It turns out that we can approach the structure and development of scales and teeth (and hair and, for that matter, feathers, and even limbs) as if the three components of the odontode were three separable evolutionary modules [4]. These modules interact, but they are indeed separable and have often been separated in the course of evolution. This point is worth emphasizing because it is frequently forgotten in reviews of scale/tooth evolution, which tend to treat the usual three-part odontode system as if it were a fundamental unit of development. Counterexamples are not hard to find. Almost all early integumentary systems have some kind of laminar bone or cartilage as a base layer. However, this is not true of more derived structures of the same class, such as feathers [W+04], or the elasmoid scales of most living actinopterygians [SA04]. Galeaspid scales have a base of laminar bone, as well as an enameloid cap, but lack any trace of a dentine-covered pulp cavity [J96]. Psammosteid heterostracans have basal bone and dentine-covered odontodes, but lack enamel, or any other hypermineralized surface layer [T64].
Thus, in all probability we can treat these components separately, because they are, in fact, three different structures which have evolved in somewhat different ways and at rather different rates. Nevertheless, we will begin with a general discussion of tooth development in the most highly derived systems. As we will discuss a little later on, denticle formation on the gill arches predated the time that the mandibular arch was reconfigured as a jaw. However, we know little about those systems and will concentrate on phylogenetically uninteresting, but better-understood, systems such as the mouse. This is followed by somewhat more specific consideration of the enamel and dentine compartments. For our purposes, we will ignore the attachment module. It is quite variable and apparently shows little phylogenetic consistency [S+98]. We will then look at some aspects the evolution of this system, followed by a brief review of some "scale theory."
 Neurulation
is the process by which vertebrates form the "neural tube" which
eventually differentiates into the spinal chord and related structures.
The presumptive neural tube tissues consist of a layer of ectoderm along the
dorsal midline of the embryo, between the notochord and an outer layer of
epidermis. The underlying notochord produces the well-known transcription
factor, Sonic hedgehog ("Shh") [5].
The overlying epidermis secretes another factor, BMP4. Under the influence
of this Shh/BMP4 gradient, the neural ectoderm thickens, elongates, and rolls up
into a tube.
Neurulation
is the process by which vertebrates form the "neural tube" which
eventually differentiates into the spinal chord and related structures.
The presumptive neural tube tissues consist of a layer of ectoderm along the
dorsal midline of the embryo, between the notochord and an outer layer of
epidermis. The underlying notochord produces the well-known transcription
factor, Sonic hedgehog ("Shh") [5].
The overlying epidermis secretes another factor, BMP4. Under the influence
of this Shh/BMP4 gradient, the neural ectoderm thickens, elongates, and rolls up
into a tube.
The developmental hall mark of the vertebrates is neural crest ectomesenchyme. As the neural tube closes, the ectodermal cells continue to grow and divide very rapidly. Many of these cells detach from the tissue matrix and become nomadic -- that is, they form an ectoderm-derived mesenchyme, or ectomesenchyme. This neural crest mesenchyme migrates down the sides of the embryo, beneath the epidermis, to locations where it differentiates into a wide variety of structures. This wandering population of neural crest cells is quite large, particularly in the head region. The patterns of mesenchyme migration are quite specific. The cells migrate from specific portions of the neural crest to specific destinations. Many are apparently already terminally committed to the tissues they will form on arrival. In addition, they act as messengers, bearing molecular orders to the mesoderm and endoderm which they contact on arrival.
 It
may be useful at this point, although perhaps not strictly necessary, to review
the basic pattern of vertebrate cranial segmentation during development.
The neural tube of the body tends to segment in an orderly way along the
boundaries between somites. In the head, there are no somites, and the
situation appears chaotic. The neural tube is broad and divides flaccidly into three parts: an anterior prosencephalon, a middle mesencephalon, and a
posterior rhombencephalon. The rhombencephalon then subdivides into 7 or 8
rhombomeres. These ectodermal neural tube "segments" are
superimposed on separate "segments" of mesodermal somitomeres and
endodermal branchiomeres (the developing jaw, hyoid, and gill regions).
This unwieldy and complex interrelationship of patterns invariably confuses
humans, but the neural crest mesenchyme cells navigate this weird landscape with ease, passing
from specific brain segments or rhombomeres to specific ventral destinations [6].
It
may be useful at this point, although perhaps not strictly necessary, to review
the basic pattern of vertebrate cranial segmentation during development.
The neural tube of the body tends to segment in an orderly way along the
boundaries between somites. In the head, there are no somites, and the
situation appears chaotic. The neural tube is broad and divides flaccidly into three parts: an anterior prosencephalon, a middle mesencephalon, and a
posterior rhombencephalon. The rhombencephalon then subdivides into 7 or 8
rhombomeres. These ectodermal neural tube "segments" are
superimposed on separate "segments" of mesodermal somitomeres and
endodermal branchiomeres (the developing jaw, hyoid, and gill regions).
This unwieldy and complex interrelationship of patterns invariably confuses
humans, but the neural crest mesenchyme cells navigate this weird landscape with ease, passing
from specific brain segments or rhombomeres to specific ventral destinations [6].
Now, while all this dorsal to ventral activity has been going on, the endodermal gut has been continuing its stately progress through the center, from posterior to anterior, forming embryonic gill pouches and other dull, but essential structures of proper embryonic administration. However, when the endoderm reaches the future mouth region, it finds itself blocked by an unruly mass of oral epithelium, the stomodeum. The two opposing forces butt up against each other and form a buccopharyngeal membrane. Behind this protective wall, the endoderm holds intense, delicate discussions with the branchial neural crest to negotiate the definitive boundaries of the branchial arches, including the jaws. As it turns out, the boundaries are established largely by reference to the Hox messages carried by the mesenchyme from the neural crest rhombomeres. But, just as order is established, the stomodeum bursts inward like an army of clowns with cream pies, covering the mandibular arch -- in fact the whole buccopharyngeal region -- with messy and complicated layers of oral epithelium, mesenchyme from the anterior parts of the brain, and gut endoderm.
It is in this complex and disorderly world that the teeth are supposed to develop. The cells of the odontode are all derived from the same population of dermal ectomesenchyme cells derived from the neural crest. More specifically the future dental cells are derived from the mesenchyme of the first rhombomere and anterior brain, at least some of which were introduced from the stomodeum. Because of their anterior origin, they are Hox-less. This appears to be essential for tooth formation in normal development. [C+02]. These cells include the dentine-producing odontoblasts of the cone, as well as the cells producing the ligaments and bone of attachment [S+98].
Odontodes develop through a variety of interactions between the epithelium --
usually, but not always, of ectodermal origin -- and underlying neural crest
ectomesenchyme. In the case of teeth, the initial signal is, as one might expect, given by the
stomodeal epithelium. In mammals, the stomodeal invasion establishes a
gradient, with FGF8 expression over the presumptive molars and BMP4 over
the incisors. The first observable step in tooth formation, the initiation
stage, is a thickening of the epithelium and condensation of the neural crest
mesenchyme at sites of tooth formation [D02].  The epithelium seems to initiate this conversation with the mesenchyme, presumably
using some tacky, but well-rehearsed pick-up line involving its FGF8
and/or BMP4.
The epithelium seems to initiate this conversation with the mesenchyme, presumably
using some tacky, but well-rehearsed pick-up line involving its FGF8
and/or BMP4.
The BMP4 gene product, in particular, is a common early epithelial signal found in many types of epithelial-mesenchyme systems. Thus, the primary result of experimentally induced over-expression of the BMP4 gene is a variety of aberrations in hair, vibrissae, claws, teeth, and sweat glands [W+04]. In the mammalian tooth, BMP4 is used and re-used at several different points. It is probably the initial epidermal signal to the underlying ectomesenchyme in all systems. In mouse tooth, epidermal BMP4 triggers the mesenchyme to produce Msx1 (and sometimes Msx2) [W+04], just as in the development of the vertebrate limb. It also induces its own production in mesenchyme, and the interaction of these signals appears to be a continuing feature of tooth development [C+97]. However, Msx1 is a homologue of, and usually identical to, Hox7. Thus, we may wonder aloud if the dentine cone and pulp cavity are homologues of some far more ancient structure constructed on the main highway of homeobox-directed antero-posterior patterning.
However this may be, and if the mesenchyme is not too busy adjusting its make-up, it responds to the epithelial greeting with a slight up-tick in its own Pax9 production. In evoking this response, FGF is invariably more successful than BMP, which is one reason incisors are smaller than molars [8]. Thus, the initial gradient of these low level factors results in a gradient of Pax9 responses. The increase in Pax9 expression in turn seems to encourage the epithelial expression to become more discontinuous, as it concentrates its charms where they seem to be having the desired effect.
The basic picture of tooth morphogenesis is shown below, as depicted by the authors of the Gene Expression in Tooth site of the University of Helsinki.
|
Initiation stage |
Bud stage |
Cap stage |
Bell stage |
|
|
|
|
|
|
Differentiation stage |
Secretory stage crown |
Secretory stage root |
|
|
|
|
Stages of tooth development in the mouse. This image is adapted from The Gene Expression in Tooth site of the University of Helsinki. The image slices are linked to the incomparable molecular biology resources at that site. |
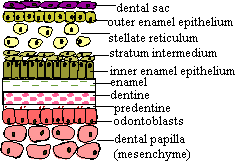
As the result of the ensuing complex conversation among transcription factors, each tooth bud forms what is called the "enamel knot" in dental systems. This is terribly misleading nomenclature, since the knot does not produce enamel. Rather it is a small group of non-dividing cells which serves as the organizer for morphological development of the tooth [J+03]. The current thinking is that the mesenchyme is ultimately persuaded to also express BMP4. Things now get rather more serious, as this regulates the production of Sonic hedgehog (Shh) which has an important role in establishing and maintaining the enamel knot in teeth and equivalent organizers structures in many other epithelial- mesenchyme systems [Z+00]. Of course, meaningless noises like "important role" are tools to avoid the dizzying view down into the vast, foaming pit of ignorance around which we are carefully treading a slippery footpath of experimental evidence. Still, this is progress.
Not surprisingly, the establishment of this organizer tends to get things organized. With the epithelial enamel knot well and truly knotted up, the transfer of inducing potential passes from the epithelium to the mesenchyme. As in all other budding relationships, he eventually runs out of rehearsed lines, and it becomes her move. Classical embryology long ago demonstrated that grafting dental epithelium onto non-dental mesenchyme could produce tooth-like structures if the experiment were performed early enough in development. Somewhat later on, this no longer works, but the reciprocal experiment (grafting dental mesenchyme onto foreign epithelium) still induces teeth [D02]. The establishment of the enamel knot at the bell stage appears to be the key event in this transition, and Shh one of the key components of the organizer.
 The organizer now kicks the morphogenetic program into gear. It is associated
with the formation of a critical boundary structure, necessary for all healthy
relationships. This is the "basement
membrane" of light microscopists. This we take to be a pseudonym of
the "basal lamina" of the electron microscopists [D+02]. This
lamina separates the dentine module from the enamel module in tooth development
and presumably mediates the interactions between them. Generally speaking,
differentiation now proceeds in both directions away from the basal
lamina, with odontoblasts creating dentine in the dental papilla (pulp
cavity) and, on a somewhat slower schedule, ameloblasts creating enamel in the
other direction [D+02]. When this process is well under way, the basal
lamina usually begins to break down. In sharks and certain teleosts, the
lamina is particularly thick and is apparently retained.
The organizer now kicks the morphogenetic program into gear. It is associated
with the formation of a critical boundary structure, necessary for all healthy
relationships. This is the "basement
membrane" of light microscopists. This we take to be a pseudonym of
the "basal lamina" of the electron microscopists [D+02]. This
lamina separates the dentine module from the enamel module in tooth development
and presumably mediates the interactions between them. Generally speaking,
differentiation now proceeds in both directions away from the basal
lamina, with odontoblasts creating dentine in the dental papilla (pulp
cavity) and, on a somewhat slower schedule, ameloblasts creating enamel in the
other direction [D+02]. When this process is well under way, the basal
lamina usually begins to break down. In sharks and certain teleosts, the
lamina is particularly thick and is apparently retained.
With the initiation of morphogenesis, the enamel knot also breaks down. BMP4-induced apoptosis may play a part in this step [J+03]. The pattern of Shh expression spreads out and/or moves to secondary organizers corresponding to individual cusps [J+00]. The pattern of cusps in the definitive tooth is primarily controlled by timing. Secondary knots which are formed earlier yield larger cusps. That is, according to current thinking, all secondary enamel knots are at least serially homologous and perhaps identical. Cusp patterns are created by differences in timing [D02], rather than under the control of Shh or some homeobox organizer. This has vast implications for mammalian tooth phylogenies, but too little is known at this point, and with too little certainty, to make much use of this tentative conclusion.
The details of further morphogenesis are considerably less general than the early stages described above. Morphogenesis of teeth, particularly in the very complex mammalian system, is also guided in part by a complex group of amelogenins and transcription factors of the Fgf, Pax, Msx, Dlx and Lef families, which seem to have considerable, but poorly understood specificity [J+03]. At any event we will now leave these teeth to develop in decent privacy and move on to other matters.
Before
going further into the subject, we need to look briefly into the
non-cellular hard stuff that is really the whole point of the exercise. The mineral component of dentine
(and enamel) may be any of several materials. Almost all are variants of apatite.
At this point, textbooks usually toss out a vague comment to the effect that
apatite is "essentially calcium phosphate," perhaps further
obscured by rote recitation of some obviously unbalanced chemical formula
for hydroxyapatite such as Ca5(PO4)3OH. The reason for this
evasive, or even disingenuous, behavior is that chemists consider apatite to be far too complex
to explain to mere bio or paleo types. To our undying embarrassment, this
humiliating assessment turns out to be correct -- if one insists on asking chemists to do the
explaining. However, we really don't need, or even want, a mental picture
of the crystal structure of apatite at 0.2 nm resolution. What we need is qualitative
information on apatite structure which explains its biological properties.
This we can supply this without resorting to Fourier
transforms, planes of symmetry, etc. See the glossary entry at apatite
for the relevant explanation. The most familiar apatite derivative is hydroxyapatite ("HAP"), the bulk mineral
constituent of bone. Chondrichthyans may produce fluorapatite, in
which a fluoride ion, rather than a hydroxyl ion, is inserted into the calcium
phosphate matrix; or they may jettison apatite altogether and substitute calcium carbonate,
CaCO3 [D+02].
Continued on Next Page
| Page Back | Unit Home | Glossary | Page Top | Page Next |
checked ATW050517
all text placed in the public domain