
| Palaeos: |  |
Thelodonti |
| The Vertebrates | Loganiidae |
| Page Back | Unit Home | Unit Dendrogram | Unit References | Taxon Index | Page Next |
| Unit Back | Vertebrates Home | Vertebrate Dendrograms | Vertebrate References | Glossary | Unit Next |
|
Abbreviated Dendrogram
Vertebrata |--Conodonta `--+--Pteraspidomorphi `--Thelodonti |--+--Furcacaudiformes | `--Thelodontida `--+--+--Katoporida | `--Cephalaspidomorphi | |--Galeaspida | `--+--Pituriaspida | |--Osteostraci | `--Gnathostomata (conventional placement) `--+--Loganiidae | |--Loganellia | `--Shielia `--Gnathostomata (alternate placement) |--Placodermi `--+--Chondrichthyes `--Teleostomi |
Contents
Overview |
Despite the rapid progress of molecular biology in recent years, the field is still in its infancy. It may yet be some time before we can say much in detail about animal development on a molecular scale. However, the knowledge that much of development is governed by gradients of signal molecules is at least as old as the classic experiments of Spemann & Mangold. Since then, we have made progress in identifying some of the signals and elucidating the genetic mechanisms which make them work. Against this background, the squamation of Loganellia deserves another look. Consider the following series of images, rearranged from the careful work of Henning Blom (1999):
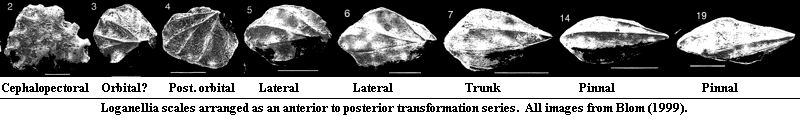
This sequence includes essentially all of the scale types found associated with Loganellia scotica. Yet, it seems intuitively clear that they can all be explained by invoking a single transformation series or, at most, a very small number of transforming gradients. The crown apex forms, becomes sharp, and moves posteriorly. The marginal crenulation deepens into continuous ridges, meeting at the apex, then flattens out and simplifies as the apex moves progressively to a more posterior position.
Thelodont scale work has focused on the enumeration of discrete scale types, since the stratigraphic utility of this information depends on the ability of workers to identify isolated scales. However, for, phylogenetic work, we seek to get as close as we can to the genetic processes which determine evolution. Usually, we are required to work with the remains of the morphological results of these processes, e.g., the size, shape, and position of an acromion process, or the presence, articulations and ornamentation of a nasal bone. Thelodonts are poor subjects for such morphological study because they have no bones and are basically amorphous. However, they may have left us something better, the actual physical trace of the developmental gradients which created their form, whatever it may have been.
As we are accustomed to morphological thinking, our natural tendency is to be frustrated by the lack of consistent morphology to observe. Recall the quotation from Van der Brugghen & Janvier (1993) near the beginning of our encounter with this taxon. Scales tell us very little about the actual shape and biology of the animal, and scale morphology is notoriously labile and subject to homoplasy. However, this frustration focuses on the end result of the developmental process. The thelodonts exhibit -- not the results of the developmental equation -- but its first derivative. For phylogenetic purposes, this may actually be a better tool, as it is one step closer to actual genetic change.
If this is a workable approach, the key would be to describe thelodont squamation in terms of the developmental gradients the observed patterns presumably reflect. Is the change in scale morphology from rostrum to caudal fin continuous? How many processes is it necessary to invoke to generate the full complement of scale morphologies? Is it necessary to invoke lateral or dorsoventral patterns? Where do these patterning influences begin and end? Do they, merely by way of example, exhibit a hox-like influence, beginning abruptly at some anteroposterior position and trailing off posteriorly?
The morphology itself may be uninformative, but the pattern by which it changes is a priori likely to reflect significant phylogenetic information. This, ultimately, may be more important to reconstructing thelodont phylogeny. ATW030622.
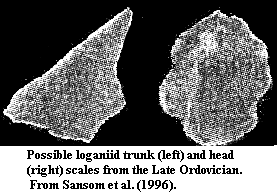 Loganiidae:
(= Loganelliidae = Loganidae) [1]. Angaralepis, Helenolepis (per
[T99], but others place it in Katoporida), Paralogania, Sandivia, Stroinolepis, Valiukia, Valyalepis.
Loganiidae:
(= Loganelliidae = Loganidae) [1]. Angaralepis, Helenolepis (per
[T99], but others place it in Katoporida), Paralogania, Sandivia, Stroinolepis, Valiukia, Valyalepis.
Range: Late Ordovician (Sandbian) to Late Silurian (Pridoli) of North America, Siberia, Greenland, Europe, Baltics, Russia
Phylogeny: Thelodonti::: Gnathostomata + *: Loganellia + Shielia.
Characters: very small denticulated plates probably derived from
pharyngeal cavity [S+96]; scale base large & bulging (???) [J96]; base
commonly has anterior prong [J96]; broad, shallow pulp depression with multiple
dentine canals emerging from it [SE02]; may lack a pulp cavity, as such, with
only a broad pulp depression; wide dentine canals leading to numerous fine
dentine tubules [S+96]; pulp cavity thin and may be restricted to posterior part
of scale [J96]; no more than one pulp canal present [M86]; bundles of dentine
tubules from cavity or cavities in the base, like mesodentine [J96]; neck short & not
 prominent [SE02]; head scale crown lateral
margins usually crenulated and grebeshki-like [SE02]; head scales crowns usually
rhomboid or oval, with anterior notches and often 1+ posterior spines [KM02]; plane of crown
at a large angle to plane of base in presumed trunk scales [S+96]; trunk scales
long [T+99].
prominent [SE02]; head scale crown lateral
margins usually crenulated and grebeshki-like [SE02]; head scales crowns usually
rhomboid or oval, with anterior notches and often 1+ posterior spines [KM02]; plane of crown
at a large angle to plane of base in presumed trunk scales [S+96]; trunk scales
long [T+99].
Note: [1] The type genus, Loganellia has had its name changed twice, as both previous names were preoccupied [MM99] [T99]. The family name "Loganellidae" is also pre-occupied by some obnoxious Cambrian trilobites who refuse to leave. Karatajute-Talimaa has consequently attempted to amend the family name to Loganelliidae. See, e.g., [MK02]. That creates an unnecessary junior synonym and invites misspelling. "Loganiidae" is a valid and well-established name. In fact, Karatajute-Talimaa established it herself in 1978. We retain it for the present.
References: Janvier (1996) [J96]; Karatajute-Talimaa & Marss (2002) [KM02]; Marss (1986) [M86]; Marss & Karatajute-Talimaa (2002) [MK02]; Miller & Marss (1999) [MM99]; Sansom & Elliott (2002) [SE02], Sansom et al. (1996) [S+96]; Turner (1999) [T99]; Turner et al. (1999) [T+99].
 |
|
Comparison of thelodontid and loganiid scales. The thelodontid scale is relatively small and has a rounded crown. It has a more distinct pulp cavity with a uniformly sharp border. The dentine tubules and canals are generally straight and there are relatively few spaces (trapped cell bodies) in the crown. This histology is characteristic of orthodentine. The base is slightly inflated and (although difficult to see in this view) tends to form a toroidal opening to the pulp cavity. The loganiid scale is larger and has a rather flat crown. The pulp cavity is barely discernable, and its margin is rough or indistinct in the central region. The dentine tubules form a messy-looking network with numerous spaces. This histology is characteristic of mesodentine. The two examples are both from Karatajute-Talimaa & Marss (2002). |
 Loganellia:
Turner, 1991 (= Logania Gross, 1967 = Loganella Turner, 1986) L.
("Thelodus") scotica Traquair, 1898;
L. asiatica; L. cuneata; L. grossi Fredholm, 1990; L.
sibirica; L. tuvaensis. Other species reassigned to Paralogania
Karatajute-Talimaa.
Loganellia:
Turner, 1991 (= Logania Gross, 1967 = Loganella Turner, 1986) L.
("Thelodus") scotica Traquair, 1898;
L. asiatica; L. cuneata; L. grossi Fredholm, 1990; L.
sibirica; L. tuvaensis. Other species reassigned to Paralogania
Karatajute-Talimaa.
Range: Late Ordovician to Late Silurian (Pridoli) of North America, Greenland, Europe, & Siberia
Phylogeny: Loganiidae: Shielia + *.
Characters: to 28 cm (30-40 cm based on isolated
tails [MR98]; cephalothorax dorsoventrally flattened [MR98]; head anteriorly
blunt [MR98]; mouth subterminal, appears as horizontal oval slit [MR98]; orbits
lateral, just behind anterolateral corners [MR98]; orbits surrounded by 2 large crescentic
scales [J96]; 7-8 branchial pouches ventral to lateral fins [J96] [MR98]; "branchial
basket" gills similar to Jamoytius [J96] [MR98]; trunk narrows
continuously behind lateral fin pair to caudal peduncle [MR98]; 1 dorsal & 1 anal fin
present, both long [J96] [MR98]; caudal forked, with larger lower lobe [J96];
slightly asymmetrical hypocercal tail [MR98]; caudal fin web with
radiating zones of enlarged scales similar to heterostracans [J96]; 22-25 such
"fin rays" [MR98]; pectoral
fins present [J96]; scales generally small [J96]; scales to 0.6 mm [MR98]; adult scales arranged in
longitudinal rows, each scale slightly overlapping scale posterior to it [MR98] [4];
base shorter than crown, quadrangular or elongate oval [MR98]; relatively
regular dentine tubules arising from  weakly
developed pulp canal or dentine canals [MR98]; scale neck found as furrows
between base & crown [MR98]; cephalopectoral scales rounded, oval,
irregular or rhomboid, with smooth, flat or convex surface [B99]; cephalopectoral
scale crowns with strong crenulation & notches arranged radially
(anteriorly) or more longitudinally (posteriorly) [B99]; more posterior
cephalopectoral scales with swollen (almost toroidal!) bases around central pulp
aperture, and crowns with elongated notches and strong posterior spine
projecting over base [B99]; orbital & anterior lateral scales round,
with moderately, high, ridged conical crown, becoming lower and with crown
becoming posterior spine in more posterior scales [B99]; trunk scales with base
somewhat anterior (without projecting past crown) and posteriorly placed pulp
cavity pore, rhomboidal crown, posterior spine and wide median furrow on a
cuneiform central area and usually 2 marginal ridges, converging on the
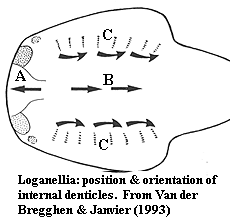
posterior crown apex [B99]; minute internal denticles in snout (A in figure)
with pointed ends anterior [VJ93] [2]; similar internal denticles in
normal orientation ("B") located centrally on head region [VJ93]
[MR98];
similar internal denticles, bases fused in linear arrays ("C"),
forming denticulated plates near/on gill arches [VJ93] [MR98] [3].
weakly
developed pulp canal or dentine canals [MR98]; scale neck found as furrows
between base & crown [MR98]; cephalopectoral scales rounded, oval,
irregular or rhomboid, with smooth, flat or convex surface [B99]; cephalopectoral
scale crowns with strong crenulation & notches arranged radially
(anteriorly) or more longitudinally (posteriorly) [B99]; more posterior
cephalopectoral scales with swollen (almost toroidal!) bases around central pulp
aperture, and crowns with elongated notches and strong posterior spine
projecting over base [B99]; orbital & anterior lateral scales round,
with moderately, high, ridged conical crown, becoming lower and with crown
becoming posterior spine in more posterior scales [B99]; trunk scales with base
somewhat anterior (without projecting past crown) and posteriorly placed pulp
cavity pore, rhomboidal crown, posterior spine and wide median furrow on a
cuneiform central area and usually 2 marginal ridges, converging on the
posterior crown apex [B99]; minute internal denticles in snout (A in figure)
with pointed ends anterior [VJ93] [2]; similar internal denticles in
normal orientation ("B") located centrally on head region [VJ93]
[MR98];
similar internal denticles, bases fused in linear arrays ("C"),
forming denticulated plates near/on gill arches [VJ93] [MR98] [3].
Notes: [1] Marss (1986) notes that the cephalopectoral and postpectoral scales of Phlebolepis are dramatically different. Does this merely indicate a more rapid transition, or was there a qualitative difference in the development of scales in Phlebolepis? [2] Van der Brugghen & Janvier [VJ93] note the similarity with "reversed" denticles in the median dorsal duct of galeaspids. Similar structures have recently been reported by Purnell (2001b) in heterostracans. So much for their phylogenetic significance... but the implication must be that they were physiologically important. Purnell notes that these tiny reversed denticles show no signs of wear in heterostracans. For aught one call tell from the microscopic micrograph in [VJ93], the same may be true in thelodonts. [3] I have read over Marss & Ritchie's [MR98] description of the branchial apparatus several times but cannot make out the full sense of it. In this section these authors also state their disagreement with Van der Brugghen & Janvier [VJ93] on the reversed orientation of certain scales. However the reversed scales observed by [VJ93] are not even in the branchial area. The problem, at a guess, is linguistic rather than scientific. Scientists are not expected to be masters of clear English exposition; but surely somebody should be editing these things. [4] based on the difference between large & small specimens, [MR98] speculate that adults were covered with closely-packed rows of scales.
Image: Life reconstruction from Museon: het populair-wetenschappelijk museum in Den Haag .
Links: Click on the fossil names above or below to find out more ...; Field Trip to the Lesmahagow Inlier; BGS Education - Fish Page 1.
References: Blom (1999) [B99]; Janvier (1996) [J96]; Marss & Ritchie (1998) [MR98]; Turner (1999) [T99]; Van der Brugghen & Janvier (1993) [VJ93]. ATW030620.
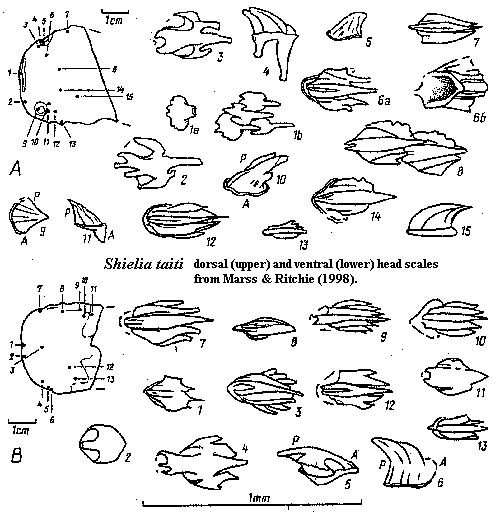 Shielia:
Marss & Ritchie, 1998; S. ("Thelodus") taiti Stetson,
1931 (= Logania taiti Gross 1967 = Loganellia taiti Turner
1991).
Shielia:
Marss & Ritchie, 1998; S. ("Thelodus") taiti Stetson,
1931 (= Logania taiti Gross 1967 = Loganellia taiti Turner
1991).
Range: Middle Silurian (Wenlock) of Europe
Phylogeny: Loganiidae: Loganellia + *.
Characters: to 13.3 cm [MR98]; entire body fusiform [MR98]; mouth terminal, horizontal slit [MR98]; orbits anterolateral, small, & surrounded by 4-5 rows of distinct tall scales, 3-5 rows of smooth scales with crenulated margins & 1-2 rows of conical scales [MR98]; 8 branchial apertures, located below pectoral fins [MR98]; single dorsal fin [MR98]; caudal fin slightly asymmetrical with longer, stronger ventral lobe & wide flexible web between lobes [MR98]; caudal fin rays absent [MR98]; flexible pectoral fins present [MR98]; paired ventral (?pelvic) fins present [MR98]; scales small (< 0.5 mm) [MR98]; scale base frequently with long anterior spur [MR98]; scale pulp cavity variable in form & size, with up to 3 pulp canals [MR98]; all pulp canals extend well into posterior part of crown [MR98]; dentine canals narrow, from pulp canal or cavity, branching terminally [MR98]; scale neck appears as shallow furrow [MR98]; variable crown forms, but strong tendency towards trilobate organization [MR98]; rostral/oral scales with flat crown projecting anteriorly over base, lateral wings, and posterior margin irregular or with 3 short spines [MR98]; more posterior cephalopectoral scales with 1-2 "wings" separated from central crown by deep furrows, becoming shallower posteriorly & laterally, ornamented by up to 5 ridges projecting over posterior end as short spines [MR98]; orbital scales high, conical & ridged [MR98]; lateral (body?) scales elongate and with fine ridges; all fins with larger & more compact (= closely spaced?) on leading edges; trailing edges of pectoral & caudal fins with very small, tripartite scales [MR98] [1]; trunk & pinnal scales imbricating [MR98];
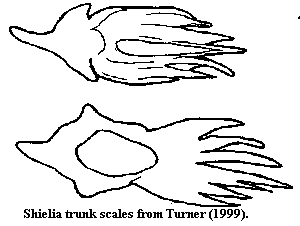
Notes: [1] perhaps very much like the unusual tripartite scale shown under Loganiidae, above.
References: Marss & Ritchie (1998) [MR98]; Turner (1999) [T99]. ATW030616.
|
|
 |
| Page Back | Unit Home | Glossary | Page Top | Page Next |
checked ATW060126