 The Call of Cthulhu
The Call of CthulhuHP Lovecraft (1927)
| Pieces | ||
| Fungi | Group I Introns |
| Fungi | ||||||
| Time |
"We live on a placid island of ignorance in the midst of black seas of infinity, and it was not meant that we should voyage far. The sciences, each straining in its own direction, have hitherto harmed us little; but some day the piecing together of dissociated knowledge will open up such terrifying vistas of reality, and of our frightful position therein, that we shall either go mad from the revelation or flee from the deadly light into the peace and safety of a new dark age."
 The Call of Cthulhu
The Call of Cthulhu
HP Lovecraft (1927)
If you have not encountered the strange world of spliceosomes and semi-autonomous RNA snippets, a quick introduction may be found at Hemiascomycetous Yeast Spliceosomal Introns. Some of the information at that site seems a bit dated, but it covers the essentials almost painlessly.
An intron, generically, is a piece of DNA which is transcribed into RNA but does not form part of the final gene product. In one fashion or another, the intron must be spliced out of the RNA before the RNA can be used for translation, ribosome formation, etc. Almost all introns are tiny and innocuous, or even beneficial, since they play a part in the regulation of transcription. They are routinely removed from the initial RNA transcript by spliceosomes and are thus known as spliceosomal introns.
However, some bacteria and basal Eukarya (Tetrahymena is the usual example) are host to Group I introns. These are a hulking, sinister lot. Group I introns are very large introns whose RNA transcript has an extremely complex secondary structure (see image at glossary entry for intron). The RNA transcript from such an intron is capable of catalyzing its own removal from the initial transcript of the host's gene. That is, it functions as a ribozyme, an enzyme made up of RNA. Most Group I introns then simply catalyze their own circularization.
Group I introns may also contain sequences coding for a homing endonuclase. Such an enzyme allows the RNA transcipt to insert itself into the DNA of an uninfected allele -- that is, into the homologous chromosome of diploid cells -- at the same or, sometimes, a different position. The cell's own DNA repair system then generates a double-stranded DNA copy of the single-stranded RNA. Alternatively, the intron may spread to new cells, or even to cells of a different organism. More commonly, Group I introns lose the ability to code homing endonucleases and rely instead on homing enzymes enzymes produced by other introns, or on association with other henchmen. Under favorable conditions they may still perform a variety of evil tasks, such as spreading through the genome somewhat in the manner of an RNA virus.
This evolutionary attempt to parasitize, not only the host cell, but also other introns, seems to have led to a gradual decline in the significance of Group I introns. As in all free rider phenomena, the most succesful parasite is the one who does the least work. However, this selects against fully competent introns so that there are fewer and fewer introns capable of expressing homing enzyme. When homing endonucleases become unavailable, Group I introns in the phylogenetic neighborhood rely exclusively on vertical transmission through the host cell line. Under those conditions, the sites responsible for interaction with the endonuclease become a neutral or negative selective factor, and the introns gradually lose even the ability to respond to homing endonuclease. At that point, it no longer serves any purpose for the intron to self-splice and circularize and the fragment ceases to be a Group I intron. Eventually, the theory goes, they are reduced to small (c. 50 bp) fragments and coopted to serve as spliceosomal introns. Thus, beginning as dangerous parasites, they end among the happy legion of the cell's own loyal regulatory agents.
At least we hope so. The picture may be complicated by, for example, the ability of homing endonuclease sequences to insert into introns previously incapable of expressing homing endonuclease. Haugen & Bhattacharya (2004).
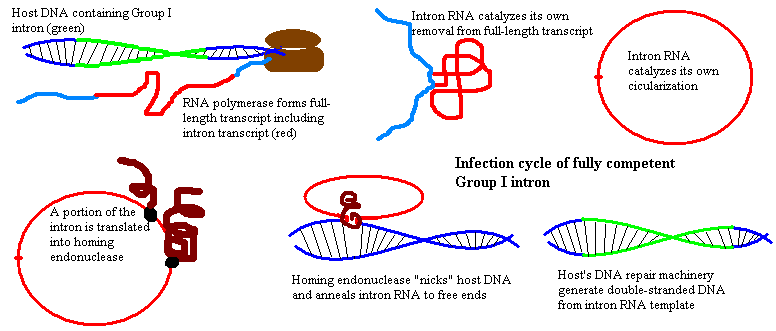
In the deep past, fully competent Group I introns were likely much more common. In fact, this may have been the way genes normally conducted business -- as semiautonomous units. But, these days, and so far as we know, Group I introns in multi-cellular organisms are rare. Those that remain have reached the stage at which they splice themselves out of the initial transcript, circularlize, then simply wait, patiently, for an ancient call which never comes, like Deep Ones awaiting the Call of Cthulhu.
Eukaryotic Group I introns are often found today in Rhodophyta and other Eukarya, largely confined to mitochondrial genes. See, e.g., Muller et al. (2001). They are most abundantly present in Fungi, particularly in yeasts. In fact, one suspects that this contributes to the yeasts' inability to form multi-cellular systems. Pezizomycotina contain relatively fewer Group I introns, but numerous spliceosomal introns -- presumably the debased remnants of a once virulent culture of parasitic Group I introns. Haugen et al. 2004).
The evolutionary implications of this theory of intron evolution are enormous. Unfortunately, they also tend to be completely speculative, pending additional work on the distribution and evolution of the type. At a minimum, Group I introns give us a possible glimpse into the nature of life before LUCA, when genes may have been semi-autonomous units. Group I introns may explain why multicellular eukaryotes were so slow to develop at all. And, since we rarely shy from speculation -- it's more fun than real work -- we also provide the following:
 Mankind, indeed all higher life, takes happy pride in that singular construct, the eukaryotic cell, whose many inner chambers are bounded by twisting membranes of alien geometry. We are accustomed to say, in our ignorance, that "this is Golgi" or "this is nucleus" while, in reality, we know nothing of the flowing, unnameable shapes which form the fundamental architecture of our own tissues. Within this labyrinth, doors and archways open suddenly, hinting of lofty passages which, however, fade like fevered dreams, leaving faceless walls with no escape. Hither and yon rush the servitors of the cell, appearing suddenly and vanishing as quickly through hitherto seamless barriers, all intent on the manifold errands of metabolism. It is well that they move briskly about their tasks. Everywhere also are guardians, vigilant agents meting out unquestioning destruction to any who deviate in the merest trifle from a rigid code of chemistry, conformation and location.
Mankind, indeed all higher life, takes happy pride in that singular construct, the eukaryotic cell, whose many inner chambers are bounded by twisting membranes of alien geometry. We are accustomed to say, in our ignorance, that "this is Golgi" or "this is nucleus" while, in reality, we know nothing of the flowing, unnameable shapes which form the fundamental architecture of our own tissues. Within this labyrinth, doors and archways open suddenly, hinting of lofty passages which, however, fade like fevered dreams, leaving faceless walls with no escape. Hither and yon rush the servitors of the cell, appearing suddenly and vanishing as quickly through hitherto seamless barriers, all intent on the manifold errands of metabolism. It is well that they move briskly about their tasks. Everywhere also are guardians, vigilant agents meting out unquestioning destruction to any who deviate in the merest trifle from a rigid code of chemistry, conformation and location.
But from whence might such a closed and closely guarded system have arisen? Earth's most ancient life, the bacterium, exhibits little need for a system such as this: one resembling a windowless mountain fastness built by a terrified populace to baffle inmates of vast intellect, but posessed of a mad and malicious spirit. What twisted genius might abide, chained and gibbering, confined within the inner fabric of our own organs, awaiting the slightest chance to bring holocaust to the higher life of this small planet?
The answer, even were it to fall withing the narrow confines of human comprehension, is surely lost forever in the gray, primordial oceans of the distant past. Yet, we may speculate; or we may do so until our minds can no longer bear the weight of the those nameless eons. It may be, for example, that those very introns of which we have lately spoken, take a not inconsiderable part on that forgotten stage. There they twist and turn, making and unmaking, in mindless dance to unheard flutes, a slithering nest of obscene genetic parasites, spawning endlessly, feeding on each other.
It is best not to consider the matter overlong. Indeed, it is best to wall such unwholesome things away in sinuous sheets of shapeless membrane, to guard scrupulously against their escape, and to allow only a few, carefully chosen products of the Crawling Chaos within to enter into the larger business of the cell. Thus the peculiar compartmentalized geometry of the eukaryotic cell, far from expressing some decent inner order and efficiency, may have evolved from antique horror and revulsion -- the final, desperate attempt to stem a swarming tide of genetic vermin at the core of our own cells.
ATW051105