Phylogeny
Systematics
References
| Rhizaria | ||
| Eukarya | Xenophyophorea |
| Eukarya | Time |
| Abbreviated Dendrogram Rhizaria
├─Radiolaria
│ ├─Acantharia
│ └─Polycystinea
└─┬─┬─Acetosporea
│ └─┬─Xenophyophorea
│ └─Foraminifera
└─┬─Phytomyxea
└─Cercozoa
|
Contents
IntroductionPhylogeny Systematics References |
 Single-celled organisms are generally required to maintain microscopic sizes. Xenophyophores, immobile shell-making mud-stickers, however, brazenly ignore all requirements of general microbial decency by attaining sizes not merely macroscopic, but positively enormous (at least by unicell standards). One of the largest species, Stannophyllum venosum Haeckel 1889, is a broad flat form up to 25 cm across, although only about a millimetre thick. Tendal 1972).
Single-celled organisms are generally required to maintain microscopic sizes. Xenophyophores, immobile shell-making mud-stickers, however, brazenly ignore all requirements of general microbial decency by attaining sizes not merely macroscopic, but positively enormous (at least by unicell standards). One of the largest species, Stannophyllum venosum Haeckel 1889, is a broad flat form up to 25 cm across, although only about a millimetre thick. Tendal 1972).
Despite such impressive dimensions, mention of them is likely to garner blank looks from most of the general public, and even from many biologists who probably should know better. This is because xenophyophores are restricted to the deep sea, not usually regarded as a prime holiday destination. Those that are occasionally pulled up from below are probably not recognised. Like benthic Steptoes, xenophyophores surround themselves with all sorts of junk they find lying around, which they use to make their shells, stuck together with a cement of polysaccharides. Id. Foraminiferan and radiolarian shells, sponge spicules, mineral grains – all are potential building materials (though individual species are often quite picky with regard to exactly what they use, and some species eschew foreign particles altogether). The particles used are referred to as xenophyae. When the fragile test is brought up, these particles tend to all fall apart, and are hence not recognised as having once been part of a larger whole.
Image: Syringammina from the web page of J. Alan Hughes.
© 2004 Christopher Taylor CT041222
The xenophyophore cell itself is organised as a series of branching tubes, which in the eternal quest for excess jargon, are referred to as granellare. The cell is multinucleate, with nuclei evenly distributed throughout the cytoplasm. The other obvious feature of the cell is the presence of numerous crystals (called granellae) of barite (BaSO4) probably secreted by the xenophyophore itself. The point of all this is unknown (Hopwood et al., 1997), though it may be to remove toxic barium solutions ingested while feeding. Tendal (1972).
Xenophyophores also produce long branching strings of faecal matter (stercomare) that are retained in the test. In some species this can make up a significant part of the test, and those species that do not collect xenophyae live out their lives in a home made entirely of their own shit.
Xenophyophores live attached to the sea-bottom, mostly above the surface except the infaunal Occultammina. They are probably suspension or filter feeders, with some extraction of food particles from the surrounding mud. Levin 1994); Riemann et al. (1993). It has been suggested that they garden microbes in the stercomare for food, but there are no actual data to support this. Xenophyophores appear to be a significant part of the benthic ecology, with large numbers of organisms living on, in and around the microenvironments created by test aggregations. Levin (1994). Beyond the production of biflagellate gametes, the reproduction of xenophyophores is still obscure, and the details have not been established by Peeping Tom biologists. Tendal (1972).
© 2004 Christopher Taylor CT041222
 The affinities of xenophyophores have generally been obscure. A large number of species were originally described by Haeckel as sponges. Other workers at the same time regarded them as agglutinated foraminifers. Other suggested relatives were slime moulds or testate amoebae currently included in Cercozoa. Tendal (1972). A recent molecular phylogeny including a single xenophyophore, Syringammina corbicula, found it nested with a fair degree of support among basal Foraminifera, amongst a clade of sessile species with agglutinated tests such as Rhizammina. Pawlowski et al. (2003).
The affinities of xenophyophores have generally been obscure. A large number of species were originally described by Haeckel as sponges. Other workers at the same time regarded them as agglutinated foraminifers. Other suggested relatives were slime moulds or testate amoebae currently included in Cercozoa. Tendal (1972). A recent molecular phylogeny including a single xenophyophore, Syringammina corbicula, found it nested with a fair degree of support among basal Foraminifera, amongst a clade of sessile species with agglutinated tests such as Rhizammina. Pawlowski et al. (2003).
It would be expected that organisms the size of xenophyophores would have an extensive fossil record. So far, though, they've got squat. This is probably due to the same problems as with recognising modern examples – like a political coalition party, xenophyophore tests are constructed of many disparate elements welded together for protection, often without anything to obviously connect them.
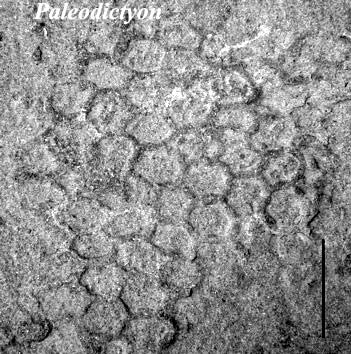 Levin (1994) describes a number of attempts to recognise fossil xenophyophores. Similarities have been noted between the pattern formed on the sediment surface by the infaunal Occultammina and the form of graphoglyptid 'traces' like Paleodictyon – suggesting that some of these may be fossil xenophyophores rather than animal feeding traces. However, graphoglyptids do not show evidence of xenophyae, and are often a lot more regular and symmetrical than expected for xenophyophores.
Levin (1994) describes a number of attempts to recognise fossil xenophyophores. Similarities have been noted between the pattern formed on the sediment surface by the infaunal Occultammina and the form of graphoglyptid 'traces' like Paleodictyon – suggesting that some of these may be fossil xenophyophores rather than animal feeding traces. However, graphoglyptids do not show evidence of xenophyae, and are often a lot more regular and symmetrical than expected for xenophyophores.
Maybury & Evans (1994) suggested that some Carboniferous fossils previously identified as phylloid 'algae' (alga – term often used by Palaeozoic palaeontologists to refer to any sessile organism that can't be made to fit anywhere else) might be xenophyophores, citing similar in structure and form, and a higher concentration of barium in the fossils than the surrounding matrix. Torres (1997) disputed this, suggesting that the similarity of structure, when looked at closely, wasn't all that obvious, and also highlighting Maybury and Evans' own caveat that the barium concentration might be the result of barium replacing calcium in preservation.
So to date, the xenophyophore fossil record is marked by a lot of wishful thinking, but few definite results – another opportunity for the coalition party analogy?
© 2004 Christopher Taylor CT041222
The Xenophyophorea, like many Eukarya, have gone by a variety of names: Arxenophyria, Domatocoela, Psamminidea, Psammininae, Xenophiophorae, Xenophyophora, Xenophyophoria, Xenophyophorida, and Xenophyophoridae.
They are rather unevenly divided between two easily distinguishable groups (Tendal, 1972). No attempt has been made to reconstruct the phylogeny of Xenophyophorea, and each of the constituent families (which are essentially form-taxa) may or may not be monophyletic. In particular, linellae are a feature unique to Stannomida among all eukaryotes, and so probably an apomorphy of them. As Psamminida are defined by the absence of linellae, it is entirely possible that it could turn out to be paraphyletic with regard to Stannomida.Psamminida – test usually rigid, without linellae. The majority of xenophyophores. Four families:
Psammettidae: Xenophyae arranged haphazardly, cemented together only at random points of contact. Test is massive, with no specialised surface layer or large openings. Psammettidae seems to be essentially defined by the absence of specialisations present in other families, and so its monophyly is particularly suspect.
Maudammina Tendal 1972
M. arenaria Tendal 1972
Homogammina Gooday & Tendal 1988
H. lamina Gooday & Tendal 1988
H. crassa Gooday 1991
H. maculosa Gooday & Tendal 1988
Psammetta Schulze 1906
P. globosa Schulze 1906
P. arenocentrum Tendal 1972
P. erythrocytomorpha Schulze 1907
P. ovale Tendal 1972
 Psamminidae: External xenophyae arranged in a distinct surface layer and/or xenophyae arranged in a number of layers. Very little cement used in test.
Psamminidae: External xenophyae arranged in a distinct surface layer and/or xenophyae arranged in a number of layers. Very little cement used in test.
Cerelpemma Laubenfels 1936
C. radiolarium (Haeckel 1889) [= Psammopemma radiolarium]
Galatheammina Tendal 1972
G. tetraedra Tendal 1972
G. calcarea (Haeckel 1889) [= Psammopemma calcareum, Cerelpemma calcareum]
G. discoveryi Gooday & Tendal 1988
G. erecta Gooday 1991
G. irregularis Gooday 1991
G. microconcha Gooday & Tendal 1988
Psammina Haeckel 1889 [incl. Psammoplakina Haeckel 1889]
P. nummulina Haeckel 1889
P. delicata Gooday & Tendal 1988
P. fusca Gooday & Tendal 1988
P. globigerina Haeckel 1889
P. plakina Haeckel 1889 [= Psammoplakina discoidea Haeckel 1889]
P. sabulosa Gooday & Tendal 1988
Reticulammina Tendal 1972 see images at Ocean Planet: Image Archive: Page 42 of 117 and George Deacon Division - DEEPSEAS Group - Images and video - Others.
R. novazealandica Tendal 1972
R. antarctica Riemann, Tendal & Gingele 1993
R. cretacea Haeckel 1889 [= Holopsamma cretaceum, Cerelpsamma cretaceum]
R. labyrinthica Tendal 1972
R. lamellata Tendal 1972
R. maini Tendal & Lewis 1978
R. plicata Gooday 1996
Syringamminidae: Test fragile, constructed of tubes of xenophyae cemented tightly together. Xenophyae restricted to tube walls, with only granellare and stercomare in the interior.
Occultammina Tendal, Swinbanks & Shirayama 1982
O. profunda Tendal, Swinbanks & Shirayama 1982
Syringammina Brady 1883 [= Arsyringammum Rhumbler 1913] See images at The Darwin Mounds - A Potential MPA.
S. fragilissima Brady 1883
S. corbicula Richardson 2001
S. minuta Pearcy 1914
S. reticulata Gooday 1996
S. tasmanensis Lewis 1966
Aschemonella Brady 1879
A. bastillensis
A. longicaudata
A. louisiana
A. ramuliformis Brady 1884
Cerelasmidae: test relatively soft, with large amounts of cement and varying amounts of xenophyae (one species, Cerelasma massa, dispenses with xenophyae altogether). Xenophyae in no obvious order, with each one fully encased in cement and not contacting any other.
Cerelasma Haeckel 1889
C. gyrosphaera Haeckel 1889
C. lamellosa Haeckel 1889
C. massa Tendal 1972
Stannomida (single family, Stannomidae) – test contains linellae, strengthening threads probably formed from mucopolysaccharides. The test is therefore much more flexible and softer than in the Psamminida. Two genera – Stannoma Haeckel, 1889 are tree-like, branching forms, while StannophyllumHaeckel, 1889 are flake- or fan-like.
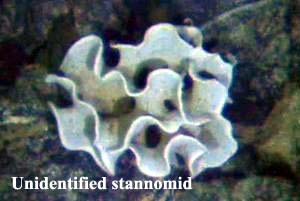 Stannomidae [= Neusinidae, Neusininae]
Stannomidae [= Neusinidae, Neusininae]
Stannophyllum Haeckel 1889 [incl. Neusina Goës 1892, Psammophyllum Haeckel 1889, Stannarium Haeckel 1889]
S. zonarium Haeckel 1889 [incl. Neusina agassizi Goës 1892, Psammophyllum annectens Haeckel 1889]
S. alatum (Haeckel 1889) [= Stannarium alatum]
S. concretum (Haeckel 1889) [= Stannarium concretum]
S. flustraceum (Haeckel 1889) [= Psammophyllum flustraceum]
S. fragilis Tendal 1972
S. globigerinum Haeckel 1889
S. granularium Tendal 1972
S. indistinctum Tendal 1972
S. mollum Tendal 1972
S. pertusum Haeckel 1889
S. radiolarium Haeckel 1889
S. reticulatum (Haeckel 1889) [= Psammophyllum reticulatum]
S. venosum Haeckel 1889
Nomen nudum: S flabellum Haeckel 1889
Stannoma Haeckel 1889 [incl. Stannoplegma Haeckel 1889]
S. dendroides Haeckel 1889
S. coralloides Haeckel 1889 [= Stannoplegma coralloides]
Xenophyophorea incertae sedis: Ammoclathrinidae. This family was described in 1889 by Haeckel (as Ammoconidae, but as this was based on a preoccupied genus name, a replacement name was supplied by Tendal, 1972) as sponges in his 'Deep-Sea Keratosa'. Ammoclathrinidae are composed of tubules that are single or branched with free or anastomosing branches. Tube walls have simple pores and are constructed of radiolarian and foraminiferan tests, sand grains and/or fragments of sponge spicules, connected by a cement of some kind. The total body is up to 20 mm in diameter. Haeckel's material is missing, and was probably destroyed over the course of his investigations. No specimens have been recorded since. The nature of Ammoclathrinidae is therefore unknown. The other 'Deep-Sea Keratosa' now comprise the xenophyophores, and the tubular form and construction from foreign particles of Ammoclathrinidae are reminiscent of xenophyophores. However, after dissolving away the calcareous material of the test of members of all three genera with acid, Haeckel recorded the presence of a possible epithelium of small granular cells, as well as small stellate cells and larger amoeboid cells. If multicellular, Ammoclathrinidae would be unlikely to be xenophyophores. For now, I include Ammoclathrinidae tentatively in the Xenophyophorea. In doing so, I am assuming that Haeckel mistook parts of a multinuclear plasmodium for separate cells, perhaps as a result of preparation effects of the acid.
Systematics References: Gooday 1991), Gooday (1996), Gooday & Tendal 1996), Levin (1994), Riemann et al. 1993), Tendal (1972).