
| Palaeos: |  |
Amniota |
| The Vertebrates | Crown Amniota |
| Page Back | Unit Home | Unit Dendrogram | Unit References | Taxon Index | Page Next |
| Unit Back | Vertebrates Home | Vertebrate Dendrograms | Vertebrate References | Glossary | Unit Next |
|
Abbreviated Dendrogram
REPTILIOMORPHA | `--AMNIOTA |--Casineria |--Westlothiana `--Crown Amniota |--Sauropsida (= Reptilia) | |--ANAPSIDA | `--EUREPTILIA `--SYNAPSIDA |
Contents
Overview |
Note: the present page is concerned with the Crown Group Amniotes, that is, the most recent common ancestor of all living amniotes and all their descendants. It is very likely however that there were reptiles, that is, amniotes, existing before then, and even that some of these are already known from the fossil record. The problem is, it is very difficult to know exactly where amongst the various transitional forms between the amphian and reptilian condition reviewed in the previous pages the ability lay eggs on dry land first appeared. But the distinction on this page is not the physiological or evolutionary between pre-amniotes and amniotes, but the phylogenetic (family history of life) distinction between the first amniotes (and associated non-amniotes) and one particular group of later (though still very early and primitive) amniotes (reptiles) that became the ancestors of all later reptiles as well as mammals and birds. MAK111121
Lungs: complex and in-folded, joined pharynx by trachea with cartilaginous support. Lungs used for CO2 dumping as well as O2 intake due to keratinized skin.
Neck: Often lengthened and more flexible.
Head: Buccal pumping eliminated, so head can be smaller and more domed.
Skull: (Captorhinid) Like "Anthracosaurs" but no otic notch or intertemporal. Postparietal, tabular and supratemporal reduced and on occipital surface only. Supraoccipital supports posterior of braincase, large stapes supports it laterally. Transverse flange on pterygoid. Elimination of large fangs. Basicranial articulation with palate moveable. Basioccipital and exoccipital form occipital condyle. Fits in ring formed intercentra and arches of atlas: ball and socket joint. Palatoquadrate reduced to quadrate and epipterygoid. Lower jaw has 1-2 coronoids and splenial.
Vertebrae: Spool-shaped centra. Small, crescentic intercentra.
Ventral axial muscles: Development of intercostals used to move ribs in respiration.
Ribs: lighter and may be joined ventrally by sternum as specialization for intercostal ventilation. Postural role assumed by epaxial muscles which are no longer primary locomotor muscles.
Limb bones: lighter – possibly reflecting proprioreceptor system. Feet used as levers for propulsion, rather than holdfasts. Ankle forms distinct hinge joint (mesotarsal). Tibiales, other bones of pes fuse to form astragalus. 23453 manus, 23454 pes.
Pelvis: Sacrum expanded from one vertebra to 2-3.
Hearing: convergent development of stapes (hyomandibula) as principal sound conduction mechanism of middle ear (i.e. connects tympanum with inner ear).
Excretion: Duct linking kidney & cloaca. Bladder not used as much for water recovery. This function tends to be performed by kidney.
Amniotic Egg: additional membranes (amnion, allantois chorion) in egg act to permit gas exchange but avoid water loss, permit large amounts of yolk storage, isolate waste products during development.
Temporal Fenestra: Synapsid at squamosal-postorbital-jugal, diapsid add at parietal-squamosal-postorbital. Euryapsid derived independently from diapsid by lack defined lower fenestra – loss of lower temporal bar. - ATW
Michel Laurin and Jacques A. Gauthier
Generations of systematists have studied amniote phylogeny at diverse genealogical levels, and until a few years ago, its broad outlines were thought to be reasonably well understood. Indeed, recognition of the major living clades, such as mammals, turtles and birds, antedates the Theory of Descent. Relations among these taxa, and especially the connections of various fossils to them, have been contentious in post-Darwinian times. Much of that controversy can, however, be attributed to the fact that during the first two-thirds of this century, there was little thought given to what constituted evidence for phylogenetic relationships. The origins of the major extant lines of Amniota have become clearer in the post-Hennigian era. Nevertheless, the precise relations of a number of clades, most notably the turtles among extant forms and the aquatic and highly divergent ichthyosaurs and sauropterygians among extinct forms, remain contentious.
Early phylogenetic analyses placed turtles outside of the remaining amniotes (only crown-clade names are listed to simplify the trees):

Gauthier et al. (1988a, b, and c) later placed turtles as the sister clade to Sauria (crown-diapsids), and this topology has now gained wide acceptance, at least among morphologists and paleontologists:
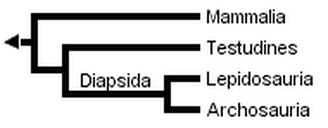
However, Rieppel (1994, 1995), Rieppel & deBraga (1996) and deBraga & Rieppel (1997) have suggested that turtles may be the sister clade to lepidosaurs. This requires that turtles are saurians who have lost both the upper and lower temporal fenestrae (holes in the skull associated with jaw muscles) so diagnostic of diapsid reptiles:

The three trees presented above include only extant taxa, and many phylogenetic analyses of amniotes have ignored extinct taxa. However, it is important to bear in mind that discovering the globally most parsimonious tree requires the inclusion of extinct taxa in a phylogenetic analysis (Gauthier et al., 1988b). Without fossils, the best-supported tree for amniotes inferred from morphological data is the following (although only one more step is required to switch the positions of lepidosaurs and turtles):
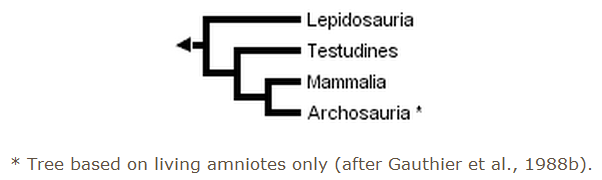
Recent molecular evidence for amniote relationships conflicts with paleontological and morphological evidence. Initially, some gene sequences suggested a close relationship between birds and mammals, although never with strong statistical support (e.g., Bishop & Friday, 1987; Goodman et al., 1987; Hedges et al., 1990). More recently, a study of the molecular evidence for the origin of birds (15 genes; 5280 nucleotides, 1461 amino acids) discovered strong support (100% bootstrap P value, BP) for a close relationship between birds and crocodilians (Hedges, 1994). A smaller data set of 11 transfer RNA genes (686 sites) also resulted in a bird-crocodilian grouping (Kumazawa & Nishida, 1995). A basal position for mammals was supported (99% BP) by analysis of a 3 kilobase portion of the mitochondrial genome containing the two ribosomal RNA genes (Hedges, 1994). In the same study, a Sphenodon-squamate relationship also was found, but support for that grouping and for the position of turtles was not very strong.
The most recent molecular phylogenies have generally placed turtles among archosauromorphs, and often within archosaurs (Mannen et al., 1997; Mannen & Li, 1999; Hedges & Poling, 1999; Hugall et al., 2007). The latter placement is the least compatible with the morphological evidence, and no convincing explanation has been found so far to explain this discrepancy.
At least two total evidence analyses (in which molecular and morphological data are combined) suggest that turtles are not diapsids (Lee, 2001; Frost et al., 2006). In both, the molecular characters are much more numerous than the morphological ones, which implies significant support for the placement of turtles outside diapsids in these molecular datasets.
Many gene sequences of birds and mammals exist, but the relatively small number of sequences from representatives of other amniote lineages, especially tuataras (Sphenodon) and turtles, has hindered the estimation of a robust molecular phylogeny for all major groups of living amniotes. This is reflected by the low resolution of the molecular phylogeny obtained by Hedges & Poling (1999) when Sphenodon (using only sequences of genes available in Sphenodon) was included:

Without Sphenodon and using the greater number of sequences available for other taxa, Hedges & Poling obtained the following fully resolved phylogeny, in which turtles are the sister-group of crocodilans:
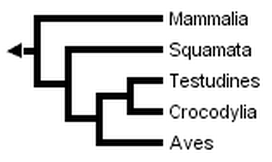
Developmental data has rarely been used to study this question, but it has recently been found to place turtles outside diapsids (Werneburg & Sánchez-Villagra, 2009).
If extinct amniotes are considered, the phylogeny is much more complex and controversial. Formerly, captorhinids were believed to be closely related to turtles (Gauthier et al., 1988b, c), but more recently, several groups of parareptiles have been sugested as closest relatives of turtles, such as procolophonids (Reisz & Laurin, 1991; Laurin & Reisz, 1995), pareiasaurs (Lee, 1993, 1994, 1995, and 1996), and Eunotosaurus (Lyson et al., 2010). Some paleontologists (Rieppel, 1994, 1995; Rieppel & deBraga, 1996; deBraga & Rieppel, 1997) place turtles among diapsids, especially as the sister-group of euryapsids (a group of Mesozoic marine diapsids). Phylogeny and Classification of Amniotes provides information about the phylogenies incorporating extinct amniote taxa, and provides a detailed classification of the relevant groups. Temporal Fenestration and the Classification of Amniotes discusses how temporal fenestration has been used to classify amniotes, and how tempororal fenestration evolved.
References
(Overworked editor's note: at some point these references need to be hyperlinked with the above, formatted (italics etc) and transferred to the reference page MAK111106)
Bishop, M. J., & A. E. Friday. 1987. Tetrapod relationships: the molecular evidence. In Patterson, C (ed.) Molecules and Morphology in Evolution: Conflict or Compromise?: 123-139. Cambridge: Cambridge University Press.
Campbell, N. A. 1993. Biology. 3rd edition. New York: The Benjamin/Cummings Publishing.
Carroll, R. L. 1964. The earliest reptiles. Zoological Journal of the Linnean Society 45: 61-83.
Coyne M. 1999. World's oldest reptile nest found. Marine Turtle Newsletter 83: 21.
deBraga M. & O. Rieppel. 1997. Reptile phylogeny and the interrelationships of turtles. Zoological Journal of the Linnean Society 120: 281-354.
Frost D. R., T. Grant, J. Faivovich, R. H. Bain, A. Haas, C. F. B. Haddad, R. O. de Sá, A. Channing, M. Wilkinson, S. C. Donnellan, C. J. Raxworthy, J. A. Campbell, B. Blotto, P. Moler, R. C. Drewes, R. A. Nussbaum, J. D. Lynch, D. M. Green, & W. C. Wheeler. 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History 297: 1–370.
Gaffney, E. S. 1980. Phylogenetic relationships of the major groups of amniotes. In A. L. Panchen (ed.) The Terrestrial Environment and the Origin of Land Vertebrates: 593-610. London: Academic Press.
Gauthier, J. A. 1994. The diversification of the amniotes. In D. R. Prothero and R. M. Schoch (ed.) Major Features of Vertebrate Evolution: 129-159. Knoxville: The Paleontological Society.
Gauthier J., R. Estes, & K. de Queiroz. 1988a. A phylogenetic analysis of Lepidosauromorpha. In: R. Estes and G. Pregill (eds.) Phylogenetic relationships of the lizard families: 15-98. Stanford: Stanford University Press.
Gauthier, J., A. G. Kluge, & T. Rowe. 1988b. Amniote phylogeny and the importance of fossils. Cladistics 4: 105-209.
Gauthier, J., A. G. Kluge, & T. Rowe. 1988c. The early evolution of the Amniota. In M. J. Benton (ed.) The phylogeny and classification of the tetrapods, Volume 1: amphibians, reptiles, birds: 103-155. Oxford: Clarendon Press.
Goodman, M., M. Miyamoto, & J. Czelusniak. 1987. Pattern and process in vertebrate phylogeny revealed by coevolution of molecules and morphologies. In C. Patterson (ed.) Molecules and Morphology in Evolution: Conflict or Compromise?: 141-176. Cambridge: Cambridge University Press.
Hedges, S. B. 1994. Molecular evidence for the origin of birds. Proceedings of the National Academy of Sciences of the United States of America 91:2621-2624.
Hedges, S. B., K. D. Moberg, & L. R. Maxson. 1990. Tetrapod phylogeny inferred from 18S and 28S ribosomal RNA sequences and a review of the evidence for amniote relationships. Molecular Biology and Evolution 7:607-633.
Hedges S. B. & L. L. Poling. 1999. A molecular phylogeny of reptiles. Science 283: 998-1001.
Hugall A. F., R. Foster, & M. S. Y. Lee. 2007. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Systematic Biology 56: 543–563.
Kumazawa, Y., & M. Nishida. 1995. Variations in mitochondrial tRNA gene organization of reptiles as phylogenetic markers. Molecular Biology and Evolution 12:759-772.
Laurin, M. & R. R. Reisz. 1995. A reevaluation of early amniote phylogeny. Zoological Journal of the Linnean Society 113: 165-223.
Laurin M. & M. Girondot. 1999. Embryo retention in sarcopterygians, and the origin of the extra-embryonic membranes of the amniotic egg. Annales des Sciences Naturelles, Zoologie, Paris, 13e SČrie 20: 99-104.
Laurin M. & R. R. Reisz. 1999. A new study of Solenodonsaurus janenschi, and a reconsideration of amniote origins and stegocephalian evolution. Canadian Journal of Earth Sciences 36: 1239-1255.
Laurin M., R. R. Reisz, & M. Girondot. 2000. Caecilian viviparity and amniote origins: a reply to Wilkinson and Nussbaum. Journal of Natural History 34: 311-315.
Lee, M., S. Y. 1993. The origin of the turtle body plan: bridging a famous morphological gap. Science 261: 1716-1720.
Lee, M. The turtle's long-lost relatives. Natural History, April 1994, 63-65.
Lee, M. S. Y. 1995. Historical burden in systematics and the interrelationships of 'Parareptiles'. Biological Reviews of the Cambridge Philosophical Society 70: 459-547.
Lee M. S. Y. 1996. Correlated progression and the origin of turtles. Nature 379: 812-815.
Lee M. S. Y. 2001. Molecules, morphology, and the monophyly of diapsid reptiles. Contributions to Zoology 70: 1-18.
Li C., X.-C. Wu, O. Rieppel, L.-T. Wang, & L.-J. Zhao. 2008. An ancestral turtle from the Late Triassic of southwestern China. Nature 456: 497–501.
Lombardi J. 1994. Embryo retention and evolution of the amniote condition. Journal of Morphology 220: 368.
Lyson T. R., G. S. Bever, B.-A. S. Bhullar, W. G. Joyce, & J. A. Gauthier. 2010. Transitional fossils and the origin of turtles. Biology Letters 6: 830–833.
Mannen H., S. C.-M. Tsoi, J. S. Krushkal, W.-H. Li, & S. S.-L. Li. 1997. The cDNA cloning and molecular evolution of reptile and pigeon lactate dehydrogenase isozymes. Molecular Biology and Evolution 14: 1081-1087.
Mannen H. & S. S.-L. Li. 1999. Molecular evidence for a clade of turtles. Molecular Phylogenetics and Evolution 13: 144-148.
Modesto S. P. 1999. Observations on the structure of the Early Permian reptile Stereosternum temidum Cope. Palaeontologia Africana 35: 7-19.
Reisz, R. R., & M. Laurin. 1991. Owenetta and the origin of turtles. Nature 349: 324-326.
Rieppel, O. 1994. Osteology of Simosaurus gaillardoti and the relationships of stem-group sauropterygia. Fieldiana Geology 1462: 1-85.
Rieppel O. 1995. Studies on skeleton formation in reptiles: implications for turtle relationships. Zoology-Analysis of Complex Systems 98: 298-308.
Rieppel O. & M. deBraga. 1996. Turtles as diapsid reptiles. Nature 384: 453-455.
Rieppel O. & R. R. Reisz. 1999. The origin and early evolution of turtles. Annual Review of Ecology and Systematics 30: 1-22.
Smithson T. R. 1989. The earliest known reptile. Nature 342: 676-678.
Smithson T. R., R. L. Carroll, A. L. Panchen, & S. M. Andrews. 1994. Westlothiana lizziae from the VisČan of East Kirkton, West Lothian, Scotland, and the amniote stem. Transactions of the Royal Society of Edinburgh 84: 383-412.
Stewart J. R. 1997. Morphology and evolution of the egg of oviparous amniotes. In: S. Sumida and K. Martin (ed.) Amniote Origins-Completing the Transition to Land (1): 291-326. London: Academic Press.
Werneburg I. & M. R. Sánchez-Villagra. 2009. Timing of organogenesis support basal position of turtles in the amniote tree of life. BMC Evolutionary Biology 9: 9 pages.
Temporal fenestration has long been used to classify amniotes (Osborn, 1903). Taxa such as Anapsida, Diapsida, Euryapsida, and Synapsida were named after their type of temporal fenestration. Temporal fenestra are large holes in the side of the skull. The function of these holes has long been debated (Case, 1924), but no consensus has been reached. Many believe that they allow muscles to expand and to lengthen. The resulting greater bulk of muscles results in a stronger jaw musculature, and the longer muscle fibers allow an increase in the gape (Pirlot, 1969).
An important distinction must be made between the type of fenestration found in certain taxa, and the taxa that were named after these types of fenestration. For instance, the anapsid condition is characterized by the lack of temporal fenestrae (Figure 1). As such, it is primitive for amniotes because all their close relatives lack temporal fenestrae. Anapsida (the taxon) includes turtles and their extinct relatives. While most members of this taxon (but not lanthanosuchids and some millerettids) have an anapsid skull, some extinct relatives of saurians (such as captorhinids and "protorothyridids") also had anapsid skulls.
|
Four main patterns of temporal fenestrae in amniote skulls a) anapsids, b) synapsids, c) diapsids), d) euryapsids. (j. jugal, p. parietal, p.o. postorbital, sq. squamosal) (Benton, 2005). original url |
The synapsid condition is characterized by the presence of a single temporal fenestra bordered minimally by the jugal, postorbital, and squamosal. The quadratojugal and the parietal occasionally contribute to the edge of this fenestra. By comparison with diapsids, this fenestra can be called a lower temporal fenestra. All early members of Synapsida, the taxon that includes mammals and their extinct relatives, had a synapsid skull, but the temporal fenestra has been drastically modified in mammals by ventral processes of the frontal and the parietal that occlude the temporal fenestra. The location of the old fenestra is still visible between the zygomatic arch, the orbit, and the dorsal part of the skull, but it is no longer a hole in the skull. A few taxa not related to Synapsida have also acquired a lower temporal fenestra. These include lanthanosuchids (Ivakhnenko, 1980) and some millerettids (members of Anapsida).
The diapsid condition is characterized by the presence of two temporal fenestrae, called the lower and the upper temporal fenestrae. The lower temporal fenestra is similar to the fenestra of synapsids, and it is generally bordered by the same bones (the jugal, postorbital, squamosal, and occasionally, the quadratojugal). The upper temporal fenestra is bordered by the postorbital, squamosal, parietal, and often, the postfrontal. This type of fenestration apparently appeared only once, in Diapsida, but it has been altered in many members of this taxon (squamates, and some early archosauromorphs, for example) by the loss of the lower temporal bar formed by the jugal and the quadratojugal.
The last type of fenestration called euryapsid has been the most problematic, partly because the origin of this condition has long been debated. It now appears that this condition is modified from the diapsid condition, and that it appeared more than once. Euryapsid skulls have only an upper temporal fenestra, usually bordered by the parietal, postfrontal, postorbital, and squamosal. Examples of euryapsid skulls include Araeoscelis, a Lower Permian araeoscelidian, placodonts, nothosaurs, and plesiosaurs (marine reptiles of the Mesozoic). Euryapsida is a taxon that includes most known euryapsids, except for Araeoscelis (Rieppel, 1993). The condition found in ichthyosaurs is sometimes distinguished from the euryapsid condition because their temporal fenestra is only bordered by the parietal, postfrontal, and supratemporal (Pirlot, 1969). This condition has been called parapsid, but it only represents a minor variation on the euryapsid pattern.
References
Carroll R. L. 1981. Plesiosaur ancestors from the Upper Permian of Madagascar. Philosophical Transactions of the Royal Society 293: 315-383.
Case E. C. 1924. A possible explanation of fenestration in the primitive reptilian skull, with notes on the temporal region of the genus Dimetrodon. Contributions from the Museum of Geology, University of Michigan 2: 1-12.
Heaton M. J. 1979. Cranial anatomy of primitive captorhinid reptiles from the Late Pennsylvanian and Early Permian Oklahoma and Texas. Bulletin of the Oklahoma Geological Survey 127: 1-84.
Ivakhnenko M. F. 1980. Lanthanosuchids from the Permian of the East European platform. Paleontological Journal 1980: 80-90.
Laurin M. & R. R. Reisz. 1995. A reevaluation of early amniote phylogeny. Zoological Journal of the Linnean Society 113: 165-223.
Osborn H. F. 1903. On the primary divison of the Reptilia into two sub- classes, Synapsida and Diapsida. Science 17: 275-276.
Pirlot P. 1969. Morphologie évolutive des chordés. Montréal: Les Presses de l'Université de Montréal.
Reisz R. R. 1981. A diapsid reptile from the Pennsylvanian of Kansas. University of Kansas Publications of the Museum of Natural History 7: 1-74.
Rieppel O. 1993. Euryapsid relationships: A preliminary analysis. Neuen Jahrbuch für Geologie und Paläontologie. Abhandlungen 188: 241- 264.
Range: from the Late Carboniferous.
Phylogeny: Cotylosauria : Diadectomorpha + Stem Amniota : ?Casineria + ? Westlothiana * : Synapsida + Sauropsida.
Characters: premaxilla with palatal, maxillary & nasal processes [MR05]; frontal contacts orbit; various patterns of fenestration related to additional musculature for jaw from dermal skull and development of musculature to supply static pressure at jaw; squamosal contributes to margin of posttemporal fenestra; hemispherical & ossified occipital condyle; pterygoid with distinct palatal surface, transverse flange and quadrate ramus [MR05]; pterygoid quadrate ramus with separate dorsal flange extending from basicranial articulation to dorsal process of quadrate, supporting elongate epipterygoid [MR05]; loss of labyrinthodont teeth, caniniform tooth present on maxilla; 2 centers of ossification in scapulocoracoid; astragalus present. Numerous additional characters listed above.
Links: Introduction to the Amniota; Amniota; Amniota.
References: Müller & Reisz (2005) [MR05]. ATW051015.
| Page Back | Unit Home | Page Top | Page Next |
checked ATW050518, new page MAK111106