
| Palaeos: |  |
The Cryogenian Period |
| Neoproterozoic | References |
| Page Back | Back: Tonian | Back: Mesoproterozoic | Up: Neoproterozoic | References |
| Page Next | Next: Ediacaran | Next: Paleozoic | Timescale |
Allen, PA & PF Hoffman (2005), Extreme winds and waves in the aftermath of a Neoproterozoic glaciation. Nature 433: 123-127.
Amthor, JE, JP Grotzinger, S Schröder, & BC Schreiber (2002), Tectonically-driven evaporite-carbonate transitions in a Precambrian/Cambrian saline giant: Ara Salt Basin of South Oman. AAPG An. Mtg. WWW. (abstr.)
Awramik, SM & JP Vanyo (1986), Heliotropism in modern stromatolites. Science 231: 1279-1281.
Bartley, JK & LC Kah (2004), Marine carbon reservoir, Corg-Ccarb coupling, and the evolution of the Proterozoic carbon cycle. Geology 32: 129-132.
Beyth, M, D Avigad H-U Wetzel, A Matthews & SM Berhe (2003), Crustal exhumation and indications for Snowball Earth in the East African Orogen: north Ethiopia and east Eritrea. Precam. Res. 123: 187–201.
Bodiselitsch, B, C Koeberl, S Master, & WU Reimold (2005), Estimating duration and intensity of Neoproterozoic snowball glaciations from Ir anomalies. Science 308: 239-242.
Bottjer, DJ, JW Hagadorn & SQ Dornbos (2000), The Cambrian substrate revolution. GSA Today 10: 1-7.
Caldeira, K & JF Kasting (1992), Susceptibility of the early Earth to irreversible glaciation caused by CO2 clouds. Nature 359: 226-228.
Canfield, DE & R Raiswell (1999), The evolution of the sulfur cycle. Amer. J. Sci. 299: 697-723.
Chakraborty, PP, A Sarkar, SK Bhattacharya & P Sanyal (2002), Isotopic and sedimentological clues to productivity change in Late Riphean sea: A case study from two intracratonic basins of India. Proc. Indian Acad. Sci. 111: 379-390.
Corsetti, FA & AJ Kaufman (1999), At least three carbon isotope excursions/glaciations in the Neoproterozoic: Carbon isotope chemostratigraphy of Neoproterozoic-Cambrian strata, southern Great Basin, USA. Ninth Annual V.M. Goldschmidt Conference. (abstr.)
Corsetti, FA, SM Awramik & D Pierce (2003), A complex microbiota from snowball Earth times: Microfossils from the Neoproterozoic Kingston Peak Formation, Death Valley, USA. Proc. Nat. Acad. Sci. USA 100: 4399-4404.
Christie-Blick, N, LE Sohl & MJ Kennedy (1999), Considering a Neoproterozoic Snowball Earth. Science 284: 1087a.
Davidson, EH, K Peterson & RA Cameron (1995), Origin of the adult bilaterian body plans: Evolution of developmental regulatory mechanisms. Science 270: 1319-1325.
Davis, SR & DM Wilkinson (2004), The conservation management value of testate amoebae as ‘restoration’ indicators: speculations based on two damaged raised mires in northwest England. The Holocene 14: 135-143.
Dornbos, SQ, DJ Bottjer & J-Y Chen (2004), Evidence for seafloor microbial mats and associated metazoan lifestyles in Lower Cambrian phosphorites of Southwest China. Lethaia 37: 127-137.
Dornbos, SQ, DJ Bottjer & J-Y Chen (2005), Paleoecology of benthic metazoans in the Early Cambrian Maotianshan Shale biota and the Middle Cambrian Burgess Shale biota: Evidence for the Cambrian substrate revolution. Palaeogeog. Palaeoclimat. Palaeoecol. 220: 47– 67.
Eiler, JM & N Kitchen (1999), Experimental study of the stable-isotope systematics of CO2 ice/vapor systems and relevance to the study of Mars. Lunar Plant. Sci. 30: 1309.
Embleton, BJJ & GE Williams (1986), Low paleolatitude of deposition for Late Precambrian periglacial varvites in South Australia – Implications for paleoclimatology. Earth Planet. Sci. Let. 79: 419-430.
Erwin, DH & EH Davidson (2002), The last common bilaterian ancestor. Development 129: 3021-3032.
Frimmel, HE (2004), Neoproterozoic sedimentation rates and timing of glaciations -- a southern African perspective in PG Eriksson, W Altermann, DR Nelson, WU Mueller & O Catuneanu [eds.], The Precambrian Earth: Tempos and Events. Elsevier.
Frimmel, HE & PG Fölling, (2004), Late Vendian closure of the Adamastor Ocean: Timing of tectonic inversion and syn-orogenic sedimentation in the Gariep Basin. Gond. Res. 7: 685-699. Folling
Goodman, JC & RT Pierrehumbert (2003), Glacial flow of floating marine ice in "Snowball Earth". J. Geophys. Res. 108: 3308.
Gould, SJ (1989), Wonderful Life. Penguin, 347 pp.
Hagadorn, JW (1998), Restriction of a Late Neoproterozoic biotope: Ediacaran faunas, microbial structures, and trace fossils from the Proterozoic-Phanerozoic transition, Great Basin, USA. Unpub. Ph.D. thesis, Univ. So'ern. Calif., 214 pp.
Halverson, GP, PF Hoffman, DP Schrag & AJ Kaufman (2002), A major perturbation of the carbon cycle before the Ghaub glaciation (Neoproterozoic) in Namibia: Prelude to snowball Earth? Geochem. Geophys. Geosys. 3(6), 10.1029/ 2001GC000244.
Hambrey, MJ & WB Harland (1981), Earth’s Pre-Pleistocene Glacial Record. Cambridge.
Harland, WB (1964), Critical evidence for a great Infra-Cambrian glaciation. Geol. Rundsch. 54: 45–61.
Harland, WB & MJS Rudwick (1964), The Great Infra-Cambrian Ice Age. Scientific American, August 1964: 28-36.
Hoffman, PF & DP Schrag (1999), Considering a Neoproterozoic Snowball Earth -- Response. Science 284: 1087a.
Hoffman, PF & DP Schrag (2000), Snowball Earth. Scientific American, January 2000, pp. 68-75.
Hoffman, PF & DP Schrag (2002), The Snowball Earth hypothesis: Testing the limits of global change. Terra Nova 14, 129–155.
Hoffman, PF, AJ Kaufman, GP Halverson, & DP Schrag (1998), A Neoproterozoic Snowball Earth. Science 281: 1342.
Holland, HD (2003), The Geologic History of Seawater, in HD Holland & KK Turekian [eds.], Treatise on Geochemistry Elsevier, 6: 583-625.
Huntley, JW, S-H Xiao, & M Kowalewski (2006), 1.3 Billion years of acritarch history: An empirical morphospace approach. Precambrian Res. 144: 52–68.
Hyde, W, TJ Crowley, SK Baum & WR Peltier (2000), Neoproterozoic ‘Snowball Earth’ simulations with a coupled climate/ice-sheet model. Nature 405: 425–429.
Kah, LC, TW Lyons & TD Frank (2004), Low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 431: 834-838.
Kaufman, AJ, AH Knoll & GM Narbonne (1997), Isotopes, ice ages, and terminal Proterozoic earth history. Proc. Natl. Acad. Sci. USA 94: 6600-6605.
Kennedy, MJ, N Christie-Blick & LE Sohl (2001), Are Proterozoic cap carbonates and isotopic excursions a record of gas hydrate destabilization following Earth's coldest intervals? Geology 29: 443-446.
Kilner, B, C Mac Niocaill & M Brasier (2005), Low-latitude glaciation in the Neoproterozoic of Oman. Geology 433: 413-416.
Kirschvink, JL (1992), Late Proterozoic low-latitude global glaciation: the Snowball Earth, in JW Schopf & C Klein [eds.] The Proterozoic Biosphere – A Multidisciplinary Study. Cambridge, pp. 51-52.
Kirschvink, JL (2002), Quand tous les Océans étaient gelés. La Recherche 355: 26-30.
Kirschvink, JL, EJ Gaidos, LE Bertani, NJ Beukes, J Gutzmer, LN Maepa, & RE Steinberger (2000), Paleoproterozoic snowball Earth: Extreme climatic and geochemical global change and its biological consequences. Proc. Nat. Acad. Sci. USA 97: 1400-1405.
Knoll, AH (1994), Proterozoic and Early Cambrian protists: Evidence for accelerating evolutionary tempo. Proc. Nat. Acad. Sci. (USA) 91: 6743-6750.
Knoll, AH (2000), Learning to tell Neoproterozoic time. Precam. Res. 100: 3-20.
Knoll, AH & SB Carroll (1999), Early animal evolution: Emerging views from comparative biology and geology. Science 284: 2129-2137.
Knoll, AH, M Walter, G Narbonne & N Christie-Blick, (2000), The Ediacaran Period: A New Addition to the Geologic Time Scale., Unpubl. Report of the Terminal Proterozoic Subcommission of the International Commission on Stratigraphy. 35 pp. (including dissenting comments) WWW (accessed 050831).
Martin, MW, DV Grazhdankin, SA Bowring, DAD Evans, MA Fedonkin, & JL Kirschvink (2000), Age of Neoproterozoic bilaterian body and trace fossils, White Sea, Russia: Implications for metazoan evolution. Science 288: 841-845.
Mayr, E (2001), What Evolution Is. Weidenfeld & Nicolson, 318 pp.
McMechan, ME (2000), Vreeland diamictites – Neoproterozoic glaciogenic slope deposits, Rocky Mountains, northeast British Columbia. Bul. Can. Pet. Geol. 48: 246-261.
Narbonne, GM (1998), The Ediacara biota: A terminal Proterozoic experiment in the evolution of life. GSA Today 8(2): 1-6.
Olcott, AN, AL Sessions, FA Corsetti, AJ Kaufman, & T Flavio de Oliviera (2005), Biomarker evidence for photosynthesis during Neoproterozoic glaciation. Science 310: 471-474.
Peterson, KJ & EH Davidson (2000), Regulatory evolution and the origins of the bilaterians. Proc. Nat. Acad. Sci. USA 97: 4430-4433.
Pierrehumbert, RT (2004), High levels of atmospheric carbon dioxide necessary for the termination of global glaciation. Nature 429: 646-649.
Pollard, D & JF Kasting (2005), Snowball Earth: A thin-ice solution with flowing sea glaciers. J. Geophy. Res. 110: C07010.
Porter, SM (2004), The fossil record of early eukaryote diversification. Pal. Soc. Papers 10: 35-50.
Porter, SM & AH Knoll (2000), Testate amoebae in the Neoproterozoic Era: Evidence from vase-shaped microfossils in the Chuar Group, Grand Canyon. Paleobiology 26: 360–385.
Poulson, CJ & RL Jacob (2004), Factors that inhibit snowball Earth simulation. Paleoceonography 19: PA4021.
Poulson, CJ, RT Pierrehumbert & RL Jacob (2001), Impact of ocean dynamics on the simulation of the Neoproterozoic "snowball Earth". Geophys. Res. Lett. 28: 1575-1578.
Rahn, T & JM Eiler (2000), Carbon isotope fractionation associated with adsorption of CO2 on mineral substrates and its relevance to the study of Mars. Lunar Planet. Sci. 31: 1933.
Rice, HN, GP Halverson & PF Hoffman (2003), Three for the Neoproterozoic: Sturtian, Marinoan and Varangerian glaciations. EGS – AGU – EUG Jt. Assem., Nice. (abstr.).
Runnegar, BN (2000), Loophole for Snowball Earth. Nature 405: 403-404.
Sankaran, AV (1999), New explanation of the geological evolution of the Indian subcontinent. Curr. Sci. 77: 331-333.
Schrag, DP, RA Berner, PF Hoffman & GP Halverson (2002), On the initiation of a snowball Earth. Geochem. Geophys. Geosys. 6: 10.1029/2001GC000219.
Schröder, S, BC Schreiber, JE Amthor, & A Matter (2004), Stratigraphy and environmental conditions of the terminal Neoproterozoic-Cambrian Period in Oman: Evidence from sulphur isotopes. J. Geol. Soc. 161: 489-499.
Socki, RA, EK Gibson, Jr., DC Golden, DW Ming, GA McKay (2003), Kinetic fractionation of stable isotopes in carbonates on Mars: Terrestrial analogs. Lunar & Planet. Sci. 34: 1938.
Sumner, DY & JP Grotzinger (2004), Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform, South Africa. Sedimentology 51: 1–27.
Sumner, DY, JL Kirschvink & BN Runnegar (1987), Soft-sediment paleomagnetic field tests of Late Precambrian glaciogenic sediments. EOS, Trans. Am. Geophys. Union 68: 1251 (abstr.).
Vanyo, JP & SM Awramik (1982), Length of day and obliquity of the ecliptic 850 MA ago: Preliminary results of a stromatolite growth model. Geophy. Res. Let. 9: 1124-1128.
Vanyo, JP & SM Awramik (1985), Stromatolites and Earth-Moon-Sun dynamics. Precam. Res., 29: 121-142.
Vanyo, JP, RA Hutchinson & SM Awramik (1986) Heliotropism in microbial stromatolitic growths at Yellowstone National Park: Geophysical inferences. EOS, Trans. Am. Geophys. Union 67: 153-156 (abstr.).
Vidal, G (1981), Aspects of problematic acid-resistant, organic-walled microfossils (acritarchs) in the Upper Proterozoic of the North Atlantic region. Precam. Res. 15: 9-23.
Walker, G (2003), Snowball Earth. Bloomsbury, 269 pp.
Williams, GE (1975), Late Precambrian glacial climate and the Earth's obliquity. Geol. Mag. 112: 441-465.
Ziegler, AM, ML Hulver, AL Lottes & WF Schmachtenberg (1984), Uniformitarianism and paleoclimates: Inferences from the distribution of carbonate rocks, in P Brenchley [ed.], Fossils and Climate. Wiley, pp. 3–27.
[1] We were embarrassed to find that there is no other full explanation of carbon isotopes in Palaeos, so here we go. We assume no background.
All ordinary materials are made up of atoms. Atoms contain a very small, very dense nucleus. The nucleus is made up of two kinds of particles: protons, which have a positive charge, and neutrons which are very similar, but have no charge. The electric charges in the nucleus do not cause the nucleus to fly apart because these nucleons (protons & neutrons) are held together by the strong nuclear force, which is stronger than electrical repulsion at very short distances. The nucleus is surrounded by a very much larger, but more diffuse, cloud of negatively-charged electrons. Each electron has exactly the same charge as a proton, although it is much less massive. This supplies overall electrical charge neutrality to the atom.
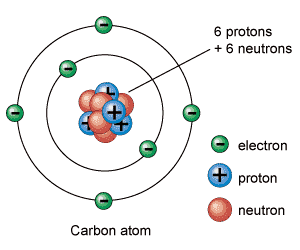 The chemical behavior
of atoms is almost (remember the almost!) completely determined by
the electron cloud, and the number of electrons is controlled by the number of
protons in the nucleus. So, any atom with 6 protons in the nucleus behaves
about the same as any other atom with the same count. Thus elements
are defined by reference to the number of protons in the nucleus, which is also
the atomic number of the element. Any atom with 6 protons is an
atom of carbon.
The chemical behavior
of atoms is almost (remember the almost!) completely determined by
the electron cloud, and the number of electrons is controlled by the number of
protons in the nucleus. So, any atom with 6 protons in the nucleus behaves
about the same as any other atom with the same count. Thus elements
are defined by reference to the number of protons in the nucleus, which is also
the atomic number of the element. Any atom with 6 protons is an
atom of carbon.
Notice we have said nothing about neutrons. For chemical purposes, they don't matter. However, only nuclei with certain numbers of neutrons are stable for each element. Thus, we only find carbon atoms with 6, 7, or 8 neutrons. These different varieties of carbon atom are referred to as isotopes. Isotopes are referred to by their total number of nucleons. Thus, the most common type of carbon has 6 protons (by definition) and 6 neutrons and is designated 12C. The isotope 13C is also common and is also stable. Carbon-14, or 14C, is created in very small quantities by nuclear reactions in the upper atmosphere involving nitrogen (element 7) and cosmic rays. 14C is not stable. One of the neutrons tends to flip back to being a proton, with the release of energy in the form of a very energetic electron. That energetic electron is one form of radioactivity. There is a 50% chance that this radioactive decay will happen to any given atom of 14C in 5730 years. Thus, we say that 14C is a radioactive isotope with a half-life of 5730 years.
In the universe as a whole, 12C makes up about 99% of all carbon, while 13C is about 1%. 14C makes up a vanishingly small fraction, which we can ignore. All atoms of carbon ought to behave in almost same way in chemical reactions. Do you still remember the "almost"? It becomes important here because it turns out that the enzymes involved in photosynthesis are a little biased. They are slightly more likely to fix CO2 molecules with 12C than we would expect by chance. Virtually all carbon in living things ultimately derives from photosynthesis. Thus almost all organic (or biogenic) carbon, carbon which is or was part of a living creature, is isotopically light, meaning it has slightly more than the usual 99% 12C. When a great deal of carbon is tied up in organic sediments, the remaining carbon in atmospheric carbon dioxide becomes isotopically heavy. This is called a positive excursion in 13C, or, for those wishing to be particularly obscure, a positive δ13C anomaly. Fortunately, it is remarkably easy to measure the ratios of carbon isotopes to very high precision using mass spectroscopy (= mass spec). By examining ancient inorganic carbon, we can read the isotopic state of the atmosphere at any given time in the past. There can be great debate about the reasons for an excursion, but the existence, and usually the magnitude, of the excursion are easily determined and quite reproducible.
Footnote to footnote: We apologize for the stupid picture. Do not get the idea that electrons orbit about the nucleus like a planet circling a star. That is completely inaccurate. Explaining why would take too long, so we'll take it up another time.
[2] Chris's essay explains the cap carbonates here. These are calcium magnesium carbonates, or dolomite [CaMg(CO3)2], associated with the end of the major glaciations, and overlying the glacial tillite (unsorted terminal glacial rock) on all continents. The cap carbonates are sometimes found without tillite, presumably deposited in deep water not actually reached by the continental glaciers.
| Page Back | Unit Home | Page Top | Page Next |