
| Palaeos |  |
Dinocaridida |
| Ecdysozoa | Dinocaridida |
| Page Back: Unit Home | Unit Up: Ecdysozoa | Unit Home | Clade Up: Panarthropoda | Page Next: Dinocaridida (2) |
| Unit Back: Onychophora | Clade Down: Arthropoda | Dendrogram | References | Unit Next: Arthropoda |
|
Abbreviated Dendrogram
PANARTHROPODA `==Siberiidae | `--DINOCARIDIDA |--Kerygmachela `--+--Pambdelurion `--+--Opabinia `--+--Anomalocarididae | |--Hurdia | `--+--Anomalocaris | `--Laggania `--+--Schinderhannes `--ARTHROPODA |
Contents
Overview |
(The first few paragraphs are adapted and modified from Wikipedia - MAK120507):
The Dinocaridida (sometimes spelled Dinocarida, but the second 'id' is linguistically correct Hou et al 2006) is a linnaean-evolutionary taxon (paraphyletic, not cladistic) of early Paleozoic arthropod-like marine animals that are best known (whether actual abundance or taphonomic bias) from the early and middle Cambrian, but continue dthrough to at least the Devonian, and very likely later. IThe name comes from Greek, "deinos" and "caris," meaning "terror shrimp" or "terror crab," due to their crustacean-like appearance and the hypotheses suggesting that members of this class were the apex predators of their time.
Dinocaridids are bilaterally symmetrical, with a non-mineralized cuticle and a body divided into two major tagmata, or body-sections. The frontal section should have one or more claws found just in front of the mouth, which is located on these creatures' underside. The body will possess thirteen or more segments, each with its own gill branch and swimming lobe. It is thought that these lobes moved in an up-and-down motion to propel the animal forward (Usami 2006); although commonly imagined as similar to the fins of a cuttlefish, the lobes were rigid and would have been more like blades of a fan or paddles.
After original uncertainty, it is now agreed that Dinocaridids are a stem group to arthropods, intermediate between Lobopodia and true arthropods.
(Most of the rest of the page is taken more or less verbatum from Chris Clowes Peripatus website, and quotes mostly from a now dated overview essay by Jeffrey Minicucci, which is for now quoted here verbatum. This material no doubt needs to be modified or added to in the light of newer discoveries and research. Additional comments by yours truely are dated accordingly. MAK120512)
"Anomalocarid arthropods have been reported from Cambrian fossil lagerstätten localities around the world. While exceptional fossil preservation, skilful preparators, and comprehensive studies have revealed much about the palaeobiology of some of these formerly enigmatic metazoans, much work still needs to be done in order properly to address the more detailed aspects of anomalocarid anatomy and the interrelationships among the genera and species placed within the family.
"The studies of the mid to late 1990s have presented us with intriguing revelations, and the most complete fossils of these animals. The level of current research and discovery is at its most promising since Whittington and Briggs (1982) pieced together the first reasonable reconstruction of Anomalocaris. Collins (1996) has provided a complete account of the history of Anomalocaris reconstructions.
"While the studies of the previous decade tended to be dominated by the publications of Whittington, Briggs, and Conway-Morris, attention in the mid to late 1990s has shifted towards three investigative ‘blocs’ represented by Hou, and Bergström; Chen, Zhou, and Ramsköld; and Collins. The studies of these competing researchers are characterized by descriptions of complete anomalocarids, identifications of new anomalocarid genera, and/or more aggressive attempts to define the family systematically (e.g. Chen et al. 1994; Collins 1996; Hou et al. 1995; Ramsköld 1995). Despite the thoroughness of the above studies, questions still remain unanswered.
"Briggs (1994) and Ramsköld (1995) are correct in demanding a phylogenetic analysis of the Anomalocaridae. Since the anomalocarid bauplan appears to be more variable than previously assumed, efforts should be directed towards compiling a new, complete list of diagnostic characters for the family. A revised list of diagnostic characters could ultimately affect the relationship between the Anomalocaridae and other possible families within Collins (1996) proposed Order Radiodonta and Class Dinocarida (although the corrected spelling is Dinocaridida). Both of these taxa are important in the effort to place anomalocarids and other similar problematic metazoans (e.g. Opabinia regalis, Kerygmachela kierkegaardi) into an eventually coherent phylogenetic context" - Minicucci 1999.
Image from Wikipedia, GNU Free Documentation/Creative Commons Attribution Share Alike |
"Using deductive logic, Rudkin (1979) postulated the existence of a large Cambrian predator responsible for inflicting wounds on individuals of the trilobite Ogygopsis klotzi. The association of this trilobite with A. canadensis grasping appendages from the Middle Cambrian Stephen Formation (Bathyuriscus-Elrathia Zone, O. klotzi faunule) prompted him to consider the enigmatic A. canadensis as the culprit. Other authors have since envisioned Anomalocaris a trilobite terror (e.g., Briggs and Mount 1982; Babcock and Robison 1989; Babcock 1993).
"In direct opposition to such studies, Hou, Bergström and Ahlberg (1995) suggest that anomalocarid mouthparts could not ‘bite off pieces of trilobites and other arthropods with a hard exoskeleton’ (p.181) and further allege that the mouthparts of previously reported anomalocarids were ‘not directed ventrally as in previous reconstructions’ (p.163). Alleged backward-facing mouthparts present in their Parapeytoia yunnanensis specimen are introduced as evidence. A major flaw in their argument is that the evidence derives from an unrepresentative sample. There are assorted complete specimens of other genera confirming the presence of mouthparts in the familiar, ventral position (see Chen et al. 1994; Collins 1996). Further, both the illustration (Hou et al. 1995, p.173, Fig. 10) and description suggest that the mouthparts of P. yunnanensis are, perhaps, different from the typical ‘Peytoia’ jaws of other anomalocarids.
"Both in appearance, and in proposed function, typical anomalocarid mouthparts must have been deadly weapons possessed of substantial cutting and crushing power. Certainly, the fact that mouthparts are more readily preserved attests to their durability. The recommendation of ... Chen and Zhou (1997) that anomalocarids should be sorted among different Dinocaridid orders because of alleged differences in mouthpart morphology is probably not warranted.
"The anomalocarid referred to as Hurdia by Collins (1992) is described as having mouthparts with an extra set of teeth that would have lined the interior of the buccal cavity (Whittington and Briggs 1985). This configuration, suggestive of a ‘pharyngeal mill’, could have been even more effective at processing hard-bodied prey" - Minicucci 1999.
"The aperture itself [of Anomalocaris] was rectangular, not circular. It could not be closed; the teeth did not meet in the middle. The jaw could be opened, however, to admit prey, and the plates could then be pulled together to draw the prey into the mouth. This would have had the effect of cracking or breaking the exoskeleton of an arthropod. Indeed, trilobites are known with healed bites in the edge of the exoskeleton that may have been made by the jaw of Anomalocaris. Some specimens of the jaw preserve additional teeth inside ..., which lined the wall of the mouth and further processed the food" (Briggs et al. 1994, pp. 201-202). According to Daley et al. 2009 p.S5, these would be Hurdia victoria, originally referred to as Anomalocaris nathorsti MAK120511
"Linear striations present on the surface of the lateral lobes of anomalocarids have been reported in several genera. Chen et al. (1994) have interpreted these features as a vein network. Hou et al. (1995) simply note them as ‘lines’. Collins (1996) suggests that A. canadensis and L. cambria possess gills on the lateral lobes, but no identifiable gill-like structures appear on the lateral lobes of his photographed specimens. Collins’ complete specimens of A. canadensis indicate a virtually ‘naked’ animal, without any surface ornamentation or raised features of any kind on the integument (pers. obs.). Examinations of L. cambria material by Whittington and Briggs (1985) suggest the equivocal presence of linear markings on the lateral lobes. Both A. saron and A. symbrachiata in Chen et al. (1994) seem to preserve traces of setae / gill-like structures (in addition to the vein network) on several of the lateral lobes. In these specimens, the alleged setae appear to lie on the ventral surface of each lateral lobe. Setae performing an alleged respiratory / gill function are usually attached to the distal edge of either a thin or paddle-shaped exopod in Cambrian arthropods and trilobites, but seeing the setae originate from the exopod surface is more complicated to explain (a similar situation exists in Opabinia regalis). In order to address the inconsistencies, we must formulate a diagnosis for correctly identifying what constitutes an anomalocarid gill. Hou and Bergström (1997) have wisely and justifiably identified problems with the fundamentally colloquial use of the term ‘gill’ in relation to descriptions of other Burgess Shale-type arthropods. If neither A. canadensis nor L. cambria possessed external gills, other locations for organs of gas exchange must be found. Further preparation of existing specimens or discoveries of new material should help to settle the issue";
"Chen et al. (1994) reported the existence of ‘two exsaggital rows of segmentally repeated ventral or internal structures of unknown function, preserved as black or light-reflective patches’ (p. 1308) in the specimens Hou et al. (1995) would later identify as A. saron and A. symbrachiata. They further allege that these nodular structures are ‘composed of bunches of fine, curved threads’ (Chen et al. 1994, p. 1305) and describe them as ‘equalling the nodular mineralized areas’ described in Anomalocaris nathorsti (p.1306). Collins (1996) mentions the same structures in his discussion of the transverse mineralized strips (lateral lobe supports) on the ventral surface of L. cambria. I believe that any attempts to draw a connection between the nodular structures identified by Chen et al. with the club-shaped structures found on the ventral surface of L. cambria are premature. Such structures appear on the lateral lobes as the terminal ends of the lateral lobe support rods. The nodular structures discussed by Chen et al. (1994) are present only on the trunk region, and are isolated from each other, not being transversely connected by mineralized strips. The possibility could exist that the nodular structures represent caeca. In Ramsköld et al. (1997), the Lower Cambrian petalopleuran xandarellid Cindarella eucalla shows evidence of caeca preserved as serially-repeated, dark stains with an ‘internal system of approximately transverse or slightly splayed tubules’ (p.29). It is tempting to compare the thread-like structures observed by Chen et al. (1994) with these" - Minicucci 1999.
"Collins’ complete specimens of A. canadensis confirm that there was no evidence of trunk annulation or external segmentation in this genus. The trunk region of A. saron in Chen et al. (1994) is described as having transverse lines, but the ‘irregularity and wrinkling of the lines’ (p.1305) suggests that these are most probably preservational folds caused during diagenensis and compaction of the carcass. Ramsköld (1997) has successfully demonstrated the existence of such misleading folds in naraoiid and tegopeltid arthropods. He has, however, hinted at the existence of certain undescribed genera with higher degrees of tagmosis possessing intersegmental trunk bars (Ramsköld 1995). A fact not discussed by researchers is the significant disparity between the virtually ‘naked’ soft-cuticle condition of A. canadensis and the high degree of tagmosis and sclerotization of P. yunanensis. According to the fossil evidence, while the most complete specimen of P. yunanensis (NIGPAS 115334) represents a substantial body moult (Hou et al. 1995), ecdysis in A. canadensis would seem to have been limited to moulting of the grasping appendages and mouthparts (Collins 1996). Persistent collecting seems to show that Royal Ontario Museum specimens of A. canadensis remains tend to consist of either a complete carcass or isolated mouthparts and grasping appendages (as seen in Collins 1996). Anything in between consists of a smeared blob (pers. obs.). As a consequence, it can be inferred that P. yunnanensis had a more significantly sclerotized cuticle. An increase in the degree of cuticle sclerotization may be correlated with an increased complexity of tagmosis in each genus"
"The high degree of anatomical detail preserved in specimens of Chengjiang anomalocarids attests to the quality of Chengjiang fossils. If the criticisms of Hou and Bergström (1997) are accepted at face value, it is arguable that the apparently smooth, featureless surface of the soft-cuticle of Collins’ Burgess Shale A. canadensis is an artefact resulting from comparably inferior preservation associated with fossils from the Burgess Shale biota. The small size of reported Chengjiang anomalocarids correlates with the assumption that their remains represent juveniles, while Collins’ described A. canadensis specimens represent adult organisms. Whether the preservational environments or different ontogenetic stages account for the differences in visible anatomical details is unclear" - Minicucci 1999.
"The ‘fantail’ reported in A. canadensis (Collins 1996), A. saron, and A. symbrachiata (Chen et al. 1994) presents a comparative problem. Phylogenetically, it is certainly not homologous with a telson, and no other arthropod has a similar posterodorsally placed structure. The presence of caudal furcae in the Chengjiang material is certainly an arthropodan character, but the ‘fantail’ complex is a uniquely derived feature. Part of the problem in adequately classifying the anomalocarids is that they developed a significant amount of derived features masking their ancestry after diverging from basal arthropods. Unclear is whether the ‘fantail’ elements were rigidly fixed in position, or moveable, like the pliable lateral lobes. If the animals could adjust their orientation with respect to the flow of water, they may have stabilized the anomalocarid body in a manner comparable to the way in which rudders stabilize ... aircraft in the air" - Minicucci 1999.
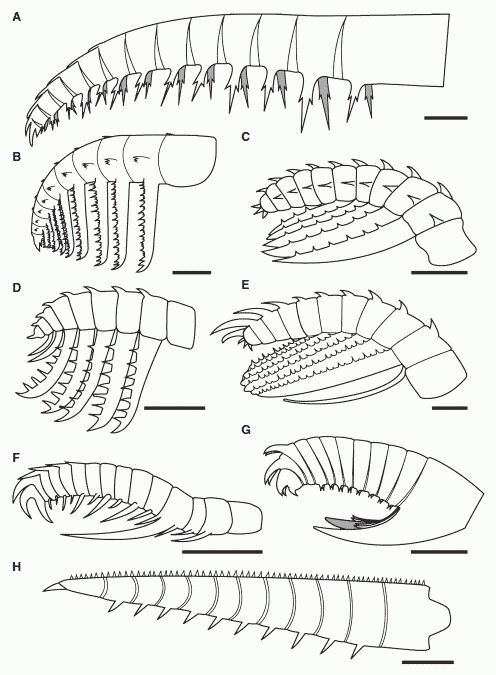 Comparative sketches of anomalocaridid frontal appendages from the Burgess Shale and the Chengjiang Fauna, showing the morphological diversity of this group . A, Anomalocaris canadensis, (modified from Briggs 1979). B, ‘Appendage F’ of Briggs 1979. C, Hurdia victoria appendage morph A (drawn from Daley et al. 2009). D, Hurdia victoria appendage morph B (drawn from Daley et al. 2009). E, ?Laggania F. Amplectobelua symbrachiata (modified from Hou et al. 1997). G, Amplectobelua stephenensis. H, Caryosyntrips serratus. All scale bars represent 10 mm. Clearly, anomalocaridids occupied different ecological niches and specialise din different prey types and feeding strategies. Drawing from Daley & Budd 2010 fig.1. Also at Amplectobelua stephenensis. Burgess Shale Fossil Gallery. Virtual Museum of Canada.;
Comparative sketches of anomalocaridid frontal appendages from the Burgess Shale and the Chengjiang Fauna, showing the morphological diversity of this group . A, Anomalocaris canadensis, (modified from Briggs 1979). B, ‘Appendage F’ of Briggs 1979. C, Hurdia victoria appendage morph A (drawn from Daley et al. 2009). D, Hurdia victoria appendage morph B (drawn from Daley et al. 2009). E, ?Laggania F. Amplectobelua symbrachiata (modified from Hou et al. 1997). G, Amplectobelua stephenensis. H, Caryosyntrips serratus. All scale bars represent 10 mm. Clearly, anomalocaridids occupied different ecological niches and specialise din different prey types and feeding strategies. Drawing from Daley & Budd 2010 fig.1. Also at Amplectobelua stephenensis. Burgess Shale Fossil Gallery. Virtual Museum of Canada.;
|
"Recent studies dramatically emphasize the variations found in frontal grasping appendages among anomalocarid genera. Described examples are the relatively stout crushing claws of A. canadensis (Collins 1996); the wicked impaling claws of A. symbrachiata; and the long, slender claws of A. saron (Chen et al. 1994; Hou et al. 1995). The morphology of the grasping appendages of L. cambria, Cassubia infercambriensis and the unknown ‘appendage F’ anomalocarid (Briggs 1979) may militate against the view that all anomalocarids were active hunters (Whittington and Briggs 1985) because such appendages could be interpreted as the instruments of sweep-feeders (see discussion by Dzik and Lendzion 1988, and Nedin 1995). The Emu Bay Shale anomalocarid Anomalocaris briggsi, known only from grasping appendages with extensive comb rows on all but the first podomere endites, also seems to be a confirmed sweep-feeder (Nedin 1995). Nedin alleges that the serrated endites on the first podomere were capable of impaling prey caught within the flexed appendage. In sharp contrast to the morphology of the fourth podomere endite on the grasping appendage of the confirmed impaler Amplectobelua symbrachiata (Chen et al. 1994), these particular endites show no appreciable increase in length relative to the lengths of the other podomere endites, casting some doubt on their effectiveness as impaling organs. The morphological differences between the grasping appendages of A. briggsi and those of other species of Anomalocaris are probably both sufficient and necessary to warrant assigning this species to a new genus. The grasping appendages of P. yunnanensis are worth mentioning because these differ significantly from those of other described anomalocarids. According to Hou et al. (1995), a complete appendage consists of five segments the lowest number yet reported in any genus. Rather than being composed of several podomeres, the proximal half of the grasping appendage consists of a long, stout podomere, and the arrangement of endites on each subsequent podomere gives the distal half of the appendage an almost chelate appearance. An approximately chelate grasping appendage has not been reported in any other anomalocarid"
"Superficially comparable are the frontal grasping appendages of the megacheiran fortiforcipid Fortiforceps foliosa (Hou and Bergström 1997; p.36, Fig. C). The functional morphology and development of the above type of appendage in anomalocarids merits further investigation. The endites are long, but the fact that there are only four probably indicates that they did not form a comb filter-feeding mechanism. Flexing the four distal podomeres would bring them into contact, making the appendages seem better suited for picking and manipulating, as opposed to squeezing and crushing using a deadly ‘bear-hug’ embrace, as suggested by the morphology of ‘typical’ predatory anomalocarid appendages." - Minicucci 1999.
Here Parapeytoia represents the possibility of an intriguing transitional form, assuming that the great appendages of dinocaridids and megacheirians are homologous MAK120511
Primitive dinocaridids such as Kerygmachelaand Pambdelurion were either blind or - perhaps more likely - had small simple eyes that did not easily appear in fossils, More advanced forms such as Opabinia and Anomalocaridids are extraordinary in possessing very large, compound eyes. This has been shown by recently discovered specimens of Anomalocaris from the Emu Bay Shale, South Australia. These fossils exhibit well-preserved bulbous stalked compound eyes, 2-3-cm in diameter, very like the eyes of modern insects. The paleontologists were able to count the density of lenses and estimate how many would have been present in the living animal. From the abstract (Paterson et al 2011) (photos here):
 Life reconstruction of Anomalocaris canadensis by Yukio Sato, showing the huge eyes, in a large animal a sign of a strongly visual preditor. This beautiful artwork appeared on the cover of Simon Conway Morris' The Crucible of Creation,
Life reconstruction of Anomalocaris canadensis by Yukio Sato, showing the huge eyes, in a large animal a sign of a strongly visual preditor. This beautiful artwork appeared on the cover of Simon Conway Morris' The Crucible of Creation,
|
"(The) preserved visual surfaces are composed of at least 16,000 hexagonally packed ommatidial lenses (in a single eye), [note: Drosophila, the common fruit fly, has about 800] rivalling the most acute compound eyes in modern arthropods [Larger specimens with correspondingly larger eyes would have had even more lenses]. The specimens show two distinct taphonomic modes, preserved as iron oxide (after pyrite) and calcium phosphate, demonstrating that disparate styles of early diagenetic mineralization can replicate the same type of extracellular tissue (that is, cuticle) within a single Burgess-Shale-type deposit. These fossils also provide compelling evidence for the arthropod affinities of anomalocaridids, push the origin of compound eyes deeper down the arthropod stem lineage, and indicate that the compound eye evolved before such features as a hardened exoskeleton. The inferred acuity of the anomalocaridid eye is consistent with other evidence that these animals were highly mobile visual predators in the water column. The existence of large, macrophagous nektonic predators possessing sharp vision—such as Anomalocaris—within the early Cambrian ecosystem probably helped to accelerate the escalatory ‘arms race’ that began over half a billion years ago" - Paterson et al 2011
It's worth comparing and contrasting the gigantic eyes of the proto-arthropod anomalocaridid with the equally huge - comparatively speaking - eyes of the advanced cephalopodian giant squid and the large (9 meter) ichthyosaur Temnodontosaurus. While each of these three animals were or are actively swimming giant preditors in their respective environments, anomalocaridids with their insect-like compound eyes hunted in the bright midwater photoic zone, whereas the other two with their large single lense camera eyes emphasise deep water and very little light. MAK120512
It has long been suggested that Anomalocaris fed on hard-bodied animals, including trilobites.
There is a Scientific American article (and also I think a more in-depth article in Paleobiology) that mentions predation on trilobites, and has a photo of a trilobite with a big bite taken out of the side. Interestingly, trilobite eyes were apparently designed to give optimal upward facing vision - so as to detect something swimming at them from above. They would not have evolved such organs were there not a pressing biological need for them.
"The Emergence of Animals" by Mark A. S. McMenamin - which features Anomalocaris on p.91 (the trilobite photo - an Olenellus robsonensis, is on the facing page). Unfortunately I did not record the issue when I photocopied the article - it is most probably late 80s, maybe 1987." MAK990601
Some Cambrian trilobites have been found with round or W-shaped "bite" marks, which were identified in shape with the mouthparts of Anomalocaris. Stronger evidence that Anomalocaris ate trilobites comes from fossilised faecal pellets, which contain trilobite parts and are so large that the anomalocarids are the only organisms large enough to have produced them.] However, since Anomalocaris lacks any mineralised tissue, it seemed unlikely that it would be able to penetrate the hard, calcified shell of trilobites. (Nedin, 1999) - Wikipedia
It has been argued that lack of wear on anomalocarid mouthparts suggests they did not come into regular contact with mineralised trilobite shells. Computer modeling of the Anomalocaris mouthparts suggests they were in fact better suited to sucking up smaller, soft-bodied organisms (and could not have been responsible for many trilobite deformations). (Hagadorn, 2009) - Wikipedia. Yet The premise that anomalocarids had external teeth only and so were only inoffensive munchers of organic detritus or plankton makes as much sense to the present author as the theory that T. rex was a wussy scavenger.
The role of anomalocaridids as superpreditors is inferred by a number of mutually reinforcing factors such as large body size, robust spinose frontal appendages, mouth with a dentate or tooth-like margin (of no use to a filter- or succtionfeeder), size and form of the mid-gut glands, large coprolites containing trilobite remains (anomalocaridids being the only known anoimals large enough to have made them), streamlined body, lateral swimming flaps and tail fan that all imply strong swimming capabilities (again, unnesscery in a filter feeder, unless it had to escape predators, a Cambrian animal of anomalocarid size was too large tpo have had preditors), and large stalked eyes located at the side of the head (Just as the tyrannosaur as slow-moving scavenger hypothesis is refuted by that animal's binocular vision (evident from the position of the eyes as indicated by the shape of the skull), something a scavenger wouldn't requireas t has is no need to judge precise distance, so a giant plankton feeder or mud grubber with no obvious predators wouldn't have any use for the sharpest eyes in the Cambrian seas. All of which adds up to a highly visual apex predator ), all provide mutually reinforcing evidence for a large animal adapted to tracking and capture of smaller animals (Paterson et al 2011), the Cambrian equivalent of a shark, a pliosaur, or a killer whale for example. Such an animal would have placed considerable selective pressures on prey that would have resulted in or contributed to an evolutionary arms race between the hunter and prey. MAK120511
"The majority of conclusions on anomalocarid functional morphology and anatomy in Hou et al. (1995) tend to be based on evidence from an unrepresentative sample. The described material of P. yunnanensis is hardly complete. Contrary to what Hou et al. (1995) clearly state in their paper, their best-preserved specimen of a single genus cannot reasonably serve as a template for redefining or dismissing the reconstructions of other anomalocarids. The ventral reconstruction (p. 180, Fig. 19) is problematical because there is no direct, conclusive evidence confirming the form of the anterior tagmata, and backward-facing position of the mouthparts and grasping appendages. It is ironic that Hou et al. choose ‘the radial arrangement of circum-oral sclerites’ (p. 163) as an homologous feature uniting aschelminthes and anomalocarids, while dismissing a virtual mountain of arthropod characters as irrelevant convergent features. Also problematical is their assertion that anomalocarids were dorsally covered in lanceolate scales. Such structures are allegedly identified in their specimens of P. yunnanensis, [they are not visible in the the photographs published in Hou et al. 1999 (figs. 77-78) - CC] Anomalocaris saron, and Cucumericrus decoratus. The scales are almost impossible to identify from the published photographs, casting doubt on the accuracy of the camera lucida drawings. The fact that Chen et al. (1994) did not observe the same markings in more complete specimens of the same genera casts even further doubt on the observations of Hou et al. (1995). The interpretation by the latter authors of ‘Peytoia nathorsti’ (= Laggania cambria of Collins 1996) assumes that USNM 274142 represents a dorsal view of the animal showing ‘transverse sets of lanceolate scales’ (Hou et al. 1995, p.179, Fig. 17A). Collins (1996) has successfully proven that this particular fossil represents a ventral view, and that the so-called ‘scales’ are better interpreted as ‘flexible rod supports of the lateral lobes’ (p. 290). Thus one key piece of evidence for the existence of dorsal lanceolate scales evaporates. Rather than being scales, the markings observed (if they truly exist) in the other above genera could be artefacts of preservation / effects of Neogene weathering, wrinkling caused by decay of the carcass, or a surface wrinkling of the integument actually present in life" - Minicucci 1999
The following was written in 1999:
"Of course it remains to be seen whether Collins’ conclusions can be supported by a comprehensive cladistic analysis. Chen and Zhou (1997) do not even believe that anomalocarids comprise a family level taxon. Curiously, they place anomalocarids at the phylum level, but without providing a formal, comprehensive diagnosis, or cladistic analysis. Of prime importance is the need to identify and trace morphological trends relating to the acquisition and loss of characters. For example, at some point in their evolution, certain groups of anomalocarids ceased developing biramous trunk appendages in favour of retaining only the lateral lobes. Interpreted in an arthropod context, this change translates into the loss of the endopod and the retention of the exopod. The assumption that the anomalocarid lateral lobe may be a true exopod is partly based on the presence of reported ventral, limb-like appendages being consistent with the gross morphology of the arthropod endopod. Some morphotypes also show an increase in their degrees of tagmosis and sclerotization, while others show the opposite" - Minicucci 1999.
In subsequent years (and even before, with the work of Graham Budd) a great deal of confusion has bene cleared up, although some questions still remain. With the exception of Hou & Bergström (2006 and other papers), all workers in this field agree that Collin's class Dinocaridida are not a distinct monophyletic clade by a paraphyletic grade leading up to a monophyletic Euarthropoda (true arthropods). As such they constitute a series of progressive stem forms (although the Linnaean rank remains unaffected; in this context the Dinocaridida are equivalent to the cynodonts betwene reptiles and mammals, or the theropod dinosaurs between reptiles and birds. Some of the following cladograms have already been discussed under the heading of lobopodia but will here be considered in the context of Dinocaridida. MAK120512
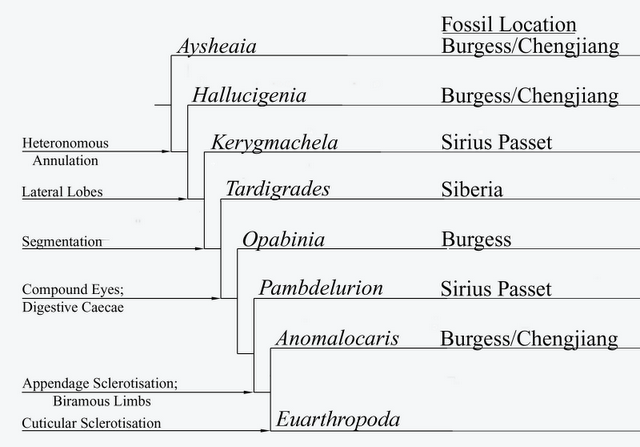
Graham Budd's scenario of panarthropod evolution, showing successive morphological innovations. This diagram, modified from Budd, 1996, 1999), is copied from Species splitting). The most basal taxa here are lobopods such as Aysheaia and Hallucinogenia, followed by a succession of dinocaridids. The very basal placement of Kerygmachela, beneath tardigrades, is due to the former's primitive features, such as a centrally located mouth (shared with most Cambrian lobopods). The current consenus is that tardigrades are a more basal panarthropod group which aquired arthropod-like characteristics by convergence. However Budd's phylogenetic position, according to which both Lobopodia and Dinocaridida constituite a single gradational series, is confirmed in all cladistic analyses |
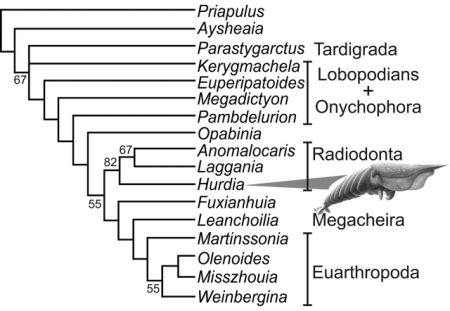
Cladistic analysis of selected stem and crown group (pan)arthropods, strict consensus of three trees, from Daley et al 2009's study of the Burgess Shale Anomalocaridid Hurdia victoria (illustrated). Although basically in agreement with Budd's earlier phylogeny, the position of Opabinia and Pambdelurion are here reversed. Hurdia, Anomalocaris, and Laggania here form a specialised side-branch and sister taxon to true arthropods beginning with Fuxianhuia. Kerygmachela is here placed even stemward of (beneath) the onychophora, based on the premise that the ventrally placed mouth (an advanced feature) only evolved once. But in view of the otherwise more advanced features of Kerygmachela, it is more parsimonious to assume that this at least happened twice, once with onychophores (perhaps an adaptation or preadaptation to terrestrial lifestyle) and once with the dinocaridid protoarthropods. Liu et al (2011)'s analysis for example place Kerygmachela above the siberiid (raptorial appendage) lobopods but beneath Pambdelurion, and we have tentatively followed that phylogeny here. |
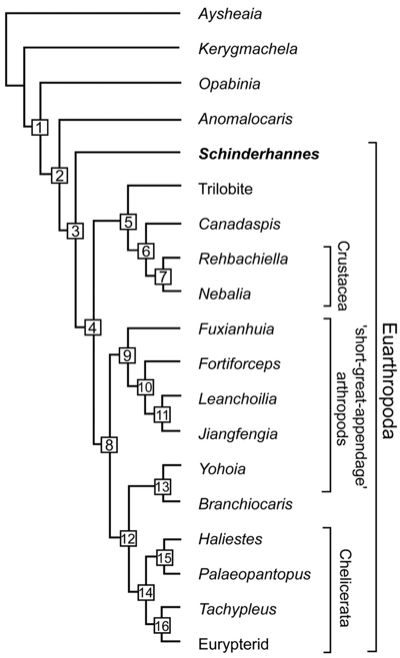
The discovery of the Devonian anomalocaridid Schinderhannes bartelsi was important not only in illustrating that the dinocaridids continued well into the Mid Palaeozoic at least, but also in the case of this advanced form provoiding a further non-,missing link between lobopods and arthropods. The cladogram here emphasises arthropods rather than panarthropods. Cladogram; tree length, 87. Consistency index, 0.5402; retention index, 0.6552. (1) Peytoia-like mouth sclerites, terminal mouth position, lateral lobes, loss of lobopod limbs, and stalked eyes. (2) Great appendages. (3) Sclerotized tergites, head shield, loss of lateral lobes, and biramous trunk appendages. (4) Stalked eyes in front and loss of radial mouth. (5) Post-antennal head appendages biramous and antenna in first head position. (6) Free cephalic carapace, carapace bivalved, and two pairs of antennae. (7) Maxilla I and II. (8) Exopods simple oval flap. (9) Two pre-oral appendages and a multisegmented trunk endopod. (10) Post-antennal head appendages biramous and tail appendages fringed with setae. (11) Long flagellae on great appendage and exopods fringed with filaments. (12) Trunk appendages uniramous and eyes not stalked. (13) No posterior tergites. (14) Tail spines and chelicere/chelifore on first head position. (15) Proboscis. (16) Six post-antennal head appendages. - from Kühl et al 2009, via PZ Myers' Pharyngula. Note that Great appendages (synapomorphy 2) are already knoown from siberiid lobopodians, and Hou & Bergström (2006) argue that anomalocaridids did have limbs (contra the claimed reversal at 1), but that these were not sclerotonised and hence not preserved. One taxon not considered in any of the above analyses is Parapeytoia, which has characteristics both of dinocaridoid protoarthropods and megacheirian "great appendage") arthropods. This ambiguity may be due to poor or partial preservation, or it may be the that Parapeytoia is a truly transitional form. If the latter, it may be either more primitive than, or more advanced than, Schinderhannes. We have tentatively placed it as more advanced, on the basis of what seems to be similarities to the great appendage arthropods. MAK120507 |
Dinocaridida Collins 1996
Range: As an evolutionary taxon Early Cambrian to Mid Devonian. As monophyletic stem + crown Arthropoda ("Paneuarthropoda" (Lieberman, 2003) or "Total Group Arthropoda" (Paterson et al 2011) from E Camb.
Phylogeny: Panarthropoda ::: Paraphyletic Siberiidae + * : Kerygmachela + (Pambdelurion + (Opabinia + (Anomalocarididae + (Schinderhannes + (Parapeytoia + Arthropoda)))))
Characters: As a paraphyletic assemblage or evolutionary grade, therefore no synapomorphies. Dinocaridid apomorphies that were previously considered unique (pineapple ring circumoral mouth plates; arthropodized frontal grasping appendages, were already acquired by the Siberiid lobopods. If used as basal taxon: $ Trunk appendage flaps in the form of lateral lobes (Edgecombe 2009) fig 3 ; Liuetal2011 fig. S1.) (this synapomorphy is lost in True Arthropods) MAK120507
Comments: As defined by Collins, Dinocaridids are bilaterally symmetrical arthropods with a body divided into two principal tagmata, recalling the prosoma and opisthosoma of chelicerates, and a non-mineralised cuticle. The front part shows no external segmentation, bears one or more pre-oral claws, one or more pairs of prominent eyes, and a ventral mouth; differing from other arthropod classes in possessing no antennae and only one appendage or pair of pre-oral appendages on the prosoma, and in bearing gilled lateral lobes on the metameric trunk. The jaws vary from none to forms with both radiating teeth and teeth in rows.
Collins included within the group the Anomalocaride (Anomalocaris and Laggania), Opabiniidae (Opabinia), Hurdia, Proboscicaris, Cassubia, and "three, possibly five, unnamed genera from China" within the Dinocarida, but was unconvinced of any close relationship between Anomalocaris and Kerygmachela. However, it is retained here for the present on account of the gill-bearing lateral lobes of the trunk. - Chris Clowes., although the central-placed mouth is a persitantly primitiv feauture already absent even in Siberiid lobopods MAK120507.
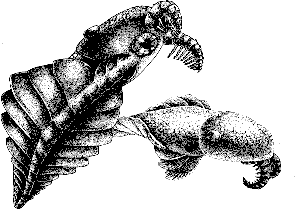
.Image: Laggania (previously classified as a species of Anomalocaris), from Wonderful Life, by Steven J Gould, artwork © Marianne Collins.
Links: Anomalocaris Homepage by Dr S.M. Gon III Best on the Web; Art Evolved - Anomalocarids; Anomalocaridid - Wikipedia MAK120507
| Page Back | Unit Home | Page Top | Page Next |
unless otherwise indicated, content © Chris Clowes 2002, & 11 Jan 2004. This page MAK120507; all content signed MAK is Creative Commons Attribution