Unless otherwise noted,
the material on this page may be used under the terms of a
Creative Commons License.
| Geological Timescale> |
||
| Timescale | Radiometric Dating |
| Stratigraphy | Time | Detailed Geological Timescale | |
| Historical discovery of Deep Time |
Units of Geological Time | Chaotian |
During the 19th century, and even well into the twentieth, geological chronology was very crude. Dates were estimated according to the supposed rate of deposition of rocks, and figures of several hundred million years were bandied out; usually arrived at through inspired guesswork rather than anything else.
With the discovery of radiometric dating, it became possible for the first time to attempt precise figures. Radiometric dating works on the principle that certain atoms and isotopes are unstable. These unstable atoms tend to "decay" into stable ones; they do this by emitting a particle or particles. This emission is what is known as radioactivity.
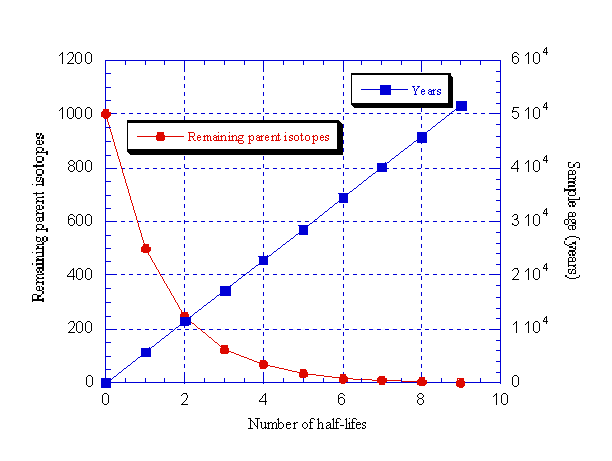
The time it takes for half of a given amount of a radioactive element to decay into a stable one is what is known as the "half-life". By matching the proportion of original unstable isotope to stable decay product, and knowing the half-life of that element, one can thus deduce the age of the rock, as shown in the following diagram. Even in the case of very long half-lives, modern scientific instruments are now accurate enough to give very fine readings.
Note: the rest of this page incorporates material from posts to the Dinosaur Mailing List circa Dec 1998.
Thank you to Brandon Haist, Allan Edels, Dann Pigdon, and Gautam Majumdar
We usually hear of Carbon 14 dating, which is very important in archaeology. The Christian Creationists have criticized it on the grounds that it is inaccurate. But these inaccuracies are the result of variation in the level of Carbon 14 in the atmosphere, and when this is worked out (through calibration with tree rings of the bristlecone pine, the oldest living organism) precise dates can be had.
The radioactive isotope Carbon 14 has a half-life of 5,730 years. This has made it useful for measuring prehistory and events occurring within the past 35 to 50 thousand years.
However, although 5730 years is the correct half-life, it is not the one used for most C-14 dating, simply because the original half-life used to determine dates back in the 1950s was wrong, and to be consistent we still tend to use the wrong value (a bit like the direction of current flow in electronics, which is the opposite of that which the electrons take, but was the original and incorrect assumption).
The practical range for dating is in the order of a few hundred to about 40,000 years BP. Any further back than that and your standard deviations go way up. Also, C-14 years do not correlate with actual calendar years, since the amount of C-14 isotopes in the atmosphere has fluctuated in the past, and the dating method assumes it was constant. Tree ring data (dendrochronology) can be used to even out this inconsistency, however the oldest trees used for calibration are in the order to about 6,000 years old, so any further back than that and you can't correct your dates (although there are reportedly some preserved huon pines in Tasmania that could take this right back to 30,000 years or so, if anyone wants to spend half their life time counting tree rings). Even if dates are corrected with tree ring data they are still not considered calendar years, but rather radiocarbon years. So a 40,000 year C-14 date and a 60,000 year thermoluminescence date could easily come from the same strata, right next to each other, and possibly reflect a date of anything between 30,000 and 70,000 calendar years depending on the standard deviations of your dates. Some thermoluminescence dates that are in the order of 50,000 years +/- 25,000 years, which with a two standard deviation limit puts it anywhere between yesterday and 100,000 years ago.
Of course C-14 would never be of any use for dating dinosaur bearing deposits, unless you want everything to date to around 40,000 years!
Radio-Carbon dating can be used for dates up to ~80,000 years ago. However, the error range increases drastically once you pass 50,000 years. Also, it is of little use in anything more recent than 5,000 years ago. (The item being tested must be organic based, and must be dead - tests on live mollusks showed an age of 2000 years).
If a fossil is completely replaced (permineralized), then it would be useless in a similar test - because it no longer is organic. Fortunately, we are able to date older fossils using the radiometric breakdown of other elements (Potassium-Argon dating, Argon-Argon dating, and Rubidium dating [I'm writing this without any refs - so this last one might be wrong]). Usually the radioactive 'clocks' for these elements are started when the elements are deposited by a volcanic eruption (usually in the form of ash). These elements have much longer half-lives than Carbon, and in some cases can be cross-referenced if more than one of these elements is present in a volcanic tuft. There are other methods for dating fossils, such as thermoluminescence.
Carbon 14's half-life is not nearly long enough to measure dates in the geological past. For that elements with a half life of many millions of years are required. Here are the half-lives of some other radioactive elements:
These are said to be used in dating techniques of gas formation light emission called thermoluminescence). Besides thermoluminescence there is also the measurement of the ratio of the radioactive material to its decay elements. For example, Uranium (U-235 or U-238) runs into the Thorium series then breakdowns into Radium and Radon, and finally, into Lead (the stable isotope).
Volcanic tuft containing U-235 also contains (stable) Lead associated directly with it. By comparing the proportion of the two, one can work out how old the deposit is. If the sample is 75% U-235 and 15% Lead (and 10% other), then the sample is approximately 300 mya. About half of the half of the original amount (1/2 * 1/2 = 1/4) of U-235 has decayed into other materials - meaning that only half of its half life has passed - therefore: ~300 mya.
Other forms of dating are:
The most common geological methods of dating are the decay of Uranium into Lead, a natural process that occurs in Uranium ore, and the Potassium-Argon method, useful with volcanic deposits.
One would such long-term dating (which is not dependent on atmospheric variations) to be totally consistent, within the limits of accuracy of the measuring instruments of course. This unfortunately is not the case. Variation is sometimes enough to make small-scale dating difficult, although never enough to make untenable the overall time-scale. Nigel Calder explains that in recent studies key dates differ by about 2 per cent [Timescale, p.233], although it sometimes seems that 5 or even 10 % may be likely. It may well be that through the formation of the rock itself, for example under conditions of varying heat and pressure, the condition of the Uranium is effected enough to throw off the dates a little. Yet even if the geological time-scale is not totally accurate, it is at least adequate to give us a fairly good chronology.
Isochron Dating - by Chris Stassen - a very useful but quite technical coverage of the whole topic of radiometric dating - from the Talks Origin archive. see also by the same author:
Radiometric Dating - A Christian Perspective - Dr. Roger C. Wiens - a very good overview. Not all Christians are Young Earth creationists!
Radiometric Dating (study notes - Pamela J. W. Gore);
Science-based dating in archaeology, by Aitken M J, Longman, London 1990, ISBN 0-582-49309-9 paperback) - describes all the dating methods available - well beyond the time period associated with archaeology.
The Handy Science Answer Book, compiled by the Science and Technology Department of the Carnegie Library of Pittsburgh
Nigel Calder - Timescale - an Atlas of the 4th dimension 1983, Hogarth Press, London
| Stratigraphy | Detailed Geological Timescale |